Results (1zkd): Difference between revisions
| Line 227: | Line 227: | ||
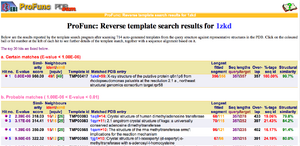
REV Reverse Template Matches (Laskowski et al, 2005) also showed probable matches for several methyltransferases (Fig.3). | |||
[[Image:Image005.png|thumb|Figure 3. REV results showing five probable matches, which are all methyl or dimethyltransferases.|center]] | |||
Revision as of 05:13, 9 June 2007
Structure of Hypothetical Protein LOC55471 Isoform 1
Comparing Structure of Proteins
Dali shows a few proteins with similar structure to 1zkd. They are 2ex4 and 1im8 which shows the highest Z-value of 11.7 and 11.6 respectively. The higher the Z-value the more significant is the results. However, they are only 10-12% identical to the query protein. Nevertheless, these 2 proteins are used to compare with the query protein as these 10-12% identity may be at the binding site or ligand which will determine the functions. 2ex4 is a human methyltransferase with S-adenosylhomocysteine and 1im8 is found to be a methyltransferase with a bound S-adenosylhomocysteine from the crystal structure of YecO from Haemophilus influenzae (HI0319).
SUMMARY: PDB/chain identifiers and structural alignment statistics
NR. STRID1 STRID2 Z RMSD LALI LSEQ2 %IDE REVERS PERMUT NFRAG TOPO PROTEIN 1: 3027-A 1zkd-A 56.8 0.0 349 349 100 0 0 1 S STRUCTURAL GENOMICS, UNKNOWN FUNCTION duf185 (rhodops 2: 3027-A 2ex4-A 11.7 3.0 185 221 12 0 0 22 S TRANSFERASE adrenal gland protein ad-003 (homo sapien 3: 3027-A 1im8-A 11.6 3.2 178 225 10 0 0 18 S TRANSFERASE yeco (methyltransferase, hypothetical pro 4: 3027-A 2gb4-A 10.8 3.3 184 231 13 0 0 19 S TRANSFERASE thiopurine s-methyltransferase (thiopurine 5: 3027-A 2fk7-A 10.7 3.8 186 277 14 0 0 19 S TRANSFERASE methoxy mycolic acid synthase 4 (mycobact 6: 3027-A 2ob1-A 10.2 3.9 196 319 9 0 0 23 S TRANSFERASE leucine carboxyl methyltransferase 1 (prot 7: 3027-A 2f8l-A 10.0 3.7 182 318 8 0 0 22 S STRUCTURAL GENOMICS, UNKNOWN FUNCTION hypothetical pro 8: 3027-A 2avn-A 10.0 3.7 183 242 11 0 0 20 S STRUCTURAL GENOMICS, UNKNOWN FUNCTION ubiquinoneMENAQU 9: 3027-A 2bzg-A 9.9 3.4 182 226 13 0 0 20 S TRANSFERASE thiopurine s-methyltransferase (thiopurine 10: 3027-A 2aot-A 9.8 4.3 182 285 13 0 0 23 S TRANSFERASE histamine n-methyltransferase (hmt) (homo
1zkd is an unknown protein, and by using proteins similar to it, the functions of this unknown protein can be predicted. With 2ex4 and 1im8 showed by Dali to be the most similar, other tools are used to determine the similarity. Combinatorial Extension Method is used. Below shows the sequence alignment and structure alignment of the unknown protein with the proteins obtained from dali:
Alignment with 2ex4 1ZKD:A 24/25 WRYXELCLGHPEHGYYV--TRDPLGREGDFTTSPEISQXFGELLGLWSASVWKAAD-EPQ 2EX4:A 24/7 IEDEKQFYS----KAKTYWKQIPPTVDGMLGGYGHISSIDINSSRKFLQRFLREGPNKTG 1ZKD:A 81/82 TLRLIEIGPGRGTXXADALRALRVLPILYQSLSVHLVEINPVLRQKQQTLLAGI-RNIHW 2EX4:A 80/64 TSCALDCGAGIGRITKRLLLPL--------FREVDMVDITEDFLVQAKTYLGEEGKRVRN 1ZKD:A 140/141 HD-----SFEDVPEGPAVILANEYFDVLPIHQAIKRETGWHERVIEIGASGELVFGVAAD 2EX4:A 132/116 YFCCGLQDFTPEPDSYDVIWIQWVIGHLT------------------------------- 1ZKD:A 195/196 PIPGFEALLPPLARLSPPGAVFEWRP--DTEILKIASRVRDQGGAALIIDYG--HLRSDV 2EX4:A 161/145 ------------------------DQHLAEFLRRCKGSL-RPNGIIVIKDNMAQE----- 1ZKD:A 251/252 GDTFQAIASHSYADPLQHPGRADLTAHV---DFDALGRAAESIGARAHGPVTQG 2EX4:A 191/175 GVILDD---------------VDSSVCRDLDVVRRIICSAG---LSLLAEERQE
Alignment with 1im8 1ZKD:A 59/60 QXFGELLGLWSASVWKAADEPQTLRLIEIGPGRGTXXADALRALRVLPILYQSLSVHLVE 1IM8:A 42/40 SNIITAIGXLAERFV-----TADSNVYDLGCSRGAATLSARRNI-----NQPNVKIIGID 1ZKD:A 119/120 INPVLRQKQQTLLAGI---RNIHWHD--SFEDVPEGPAVILANEYFDVLPIHQAIKRETG 1IM8:A 92/90 NSQPXVERCRQHIAAYHSEIPVEILCNDIRHVEIKNASXVILNFTLQFLP---------- 1ZKD:A 174/175 WHERVIEIGASGELVFGVAADPIPGFEALLPPLARLSPPGAVFEWRP--DTEILKIASRV 1IM8:A 142/140 ---------------------------------------------PEDRIALLTKIYEGL 1ZKD:A 232/233 RDQG--GAALIIDYG 1IM8:A 157/155 ---NPNGVLVLSEKF
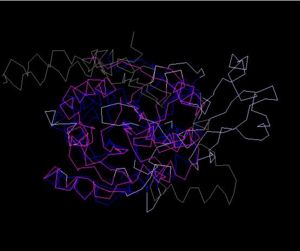
Structure Alignment with 2ex4 (Blue:1zkd, Purple: 2ex4)
Alignment Length: 294
Gaps (average per molecule): 53.5
Sequence Identity: 14.4%
RMSD min – max: 3.03A
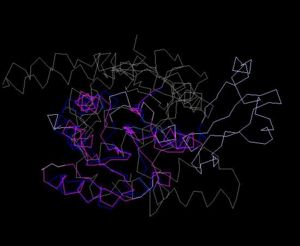
Structure Alignment with 1im8 (Blue:1zkd, Purple: 1im8)
Alignment Length: 195
Gaps (average per molecule): 38.5
Sequence Identity: 11%
RMSD min – max: 2.3A
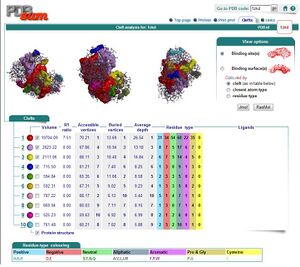
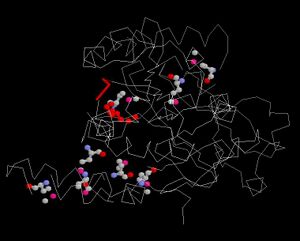
Ligand and Binding Sites
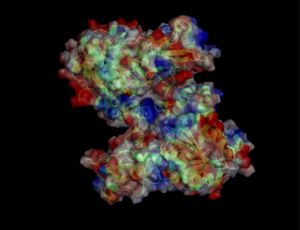
Surface Properties
Red shows negatively charge and blue shows positively charge
Domain
Domain Identification Using Pfam
DUF185: domain 1 of 1, from 64 to 299: score 227.1, E = 3.9e-65
*->alArwllveykllgyPYadlnlvElGaGrGtaielmsdlLryiarlv
+l++w + ++k+ ++P l+l E+G+GrGt +m+d+Lr+ r+
query 64 LLGLWSASVWKAADEP-QTLRLIEIGPGRGT---MMADALRA-LRVL 105
PdvyartryylvEiSprLaarQketLapkvaplGhdskveieatdlsglv
P +y+ ++++lvEi+p L+++Q++ La ++ ++
query 106 PILYQSLSVHLVEINPVLRQKQQTLLA-----------------GIR-NI 137
rWhdasileedPdgvptvliaNEVlDalPHDlvrfdkrgggwyErhVlvd
Whd s +e++P+g p v++aNE +D lP +++ +kr+ gw+Er V ++
query 138 HWHD-S-FEDVPEG-PAVILANEYFDVLP--IHQAIKRETGWHER-V-IE 180
ldgdfrlvysqeldplaglaltlreaaldPVKstkklvpsalskllpkll
+ ++lv+++++dp g+ ++l
query 181 IGASGELVFGVAADPIPGFEAL------------------------LPPL 206
ppaeevgygtEvYsParllellqalaerLpahrGrlLaiDYGhlaseyyh
+ +g+++E+ P e+l+++ + + +G++L+iDYGhl+s
query 207 ARLSPPGAVFEW-RPDT--EILKIASRVRD-QGGAALIIDYGHLRSD--- 249
prrksalaaemfngtllqayrqhahddpltnpssllVlyStvaqGlaDiT
g+++qa+ h + dpl +p G+aD+T
query 250 ------------VGDTFQAIASHSYADPLQHP------------GRADLT 275
ahVDFtalaradqyqtaakaagdlkvlgvet<-*
ahVDF+al +aa +g + + g+ t
query 276 AHVDFDALG------RAAESIG-ARAHGPVT 299
Domain DUF 185 has been identified by InterPro and Pfam as show above. In Pfam the E value of 3.9e-65 gives significant results showing that it is not by chance nor random that the match made was DUF185. However not much is known about DUF 185.
Function of Hypothetical Protein LOC55471 Isoform 1
ProFunc analysis reveals methyltransferase activity as the most likely biochemical function
By using ProFunc (Laskowski et al, 2005) the most likely biochemical function of the unknown bacterial Protein 1zkd was determined as Methyltransferase. Matching structures were determined by SSM Secondary Structure Matching (Krissinel & Henrick, 2004) showing possible matches with 9 Methyltransferases from both human and bacteria (Fig.1).
Ligand Template Matches LIG (Laskowski et al, 2005) revealed a probable match with the Protein-l-isoaspartate o-methyltransferase 1dl5 (Fig.2).
REV Reverse Template Matches (Laskowski et al, 2005) also showed probable matches for several methyltransferases (Fig.3).
Return to Report on 1zkd