Result of SNAPG: Difference between revisions
No edit summary |
No edit summary |
||
| Line 3: | Line 3: | ||
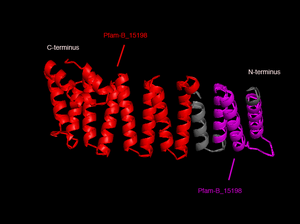
In order to analyze protein structure of SNAPG, structural comparison to known protein structure is required. An insight to SNAPG structural arrangement provides various informative data on possible protein functions and interactions with another protein and/or DNA. Based on protein families database, Pfam at Sanger, it was found that SNAPG protein matched to Pfam-B protein families and consist of 2 domains, Pfam-B_7270 (PB007270) and Pfam-B_15198 (PB015189) respectively as shown in Figure 3. Both of 2 domains appears to be associated with NSF attachment protein activity (*'''dijelasin di discussion aja!''' NSF acivity dijelasin ga?)[http://www.ebi.ac.uk/interpro/IEntry?ac=IPR000744]. | In order to analyze protein structure of SNAPG, structural comparison to known protein structure is required. An insight to SNAPG structural arrangement provides various informative data on possible protein functions and interactions with another protein and/or DNA. Based on protein families database, Pfam at Sanger, it was found that SNAPG protein matched to Pfam-B protein families and consist of 2 domains, Pfam-B_7270 (PB007270) and Pfam-B_15198 (PB015189) respectively as shown in Figure 3. Both of 2 domains appears to be associated with NSF attachment protein activity (*'''dijelasin di discussion aja!''' NSF acivity dijelasin ga?)[http://www.ebi.ac.uk/interpro/IEntry?ac=IPR000744]. | ||
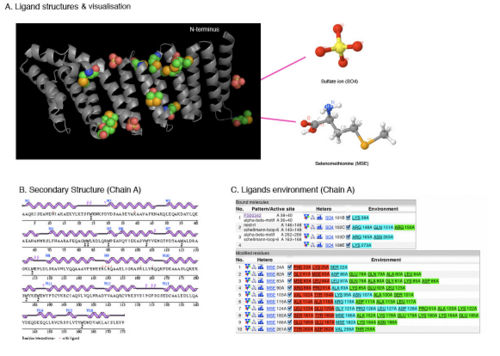
[[Image:Ligand structure visualisation.jpg|thumb|500px|left|none||Figure 1. Ligands interaction to SNAPG chain A. A) Ligands position in the structure are highlighted; B) Secondary structure of SNAPG chain A with ligands interaction indicated; and C) Various ligand interactions with SNAPG by covalent bonds (shown in red color), hydrogen bonding (cyan) and van der waals (green) ]] | |||
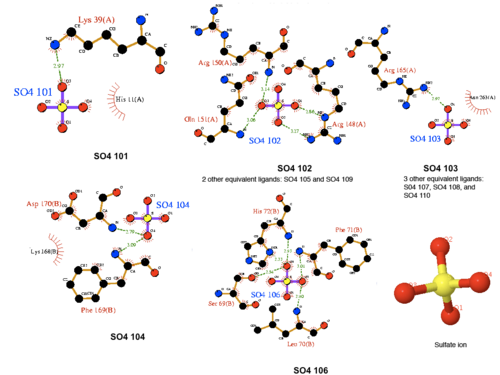
[[Image:Sulfate ion ligand interactions.png|thumb|500px|left|none|Figure 2. Various interaction of sulfanate ions]] | |||
[[Image:PFam domains.png|thumb|Figure 3. Two domains of SNAPG protein (chain A)]]. The sequence was also used against InterproScan generated by Profunc that gave an TFR (Tetratricopeptide-like helical) domain classification while that of protein family agrees with Pfam classification. | |||
[[Image:PFam domains.png|thumb|left|none|Figure 3. Two domains of SNAPG protein (chain A)]]. The sequence was also used against InterproScan generated by Profunc that gave an TFR (Tetratricopeptide-like helical) domain classification while that of protein family agrees with Pfam classification. | |||
(interproscan) | (interproscan) | ||
| Line 41: | Line 42: | ||
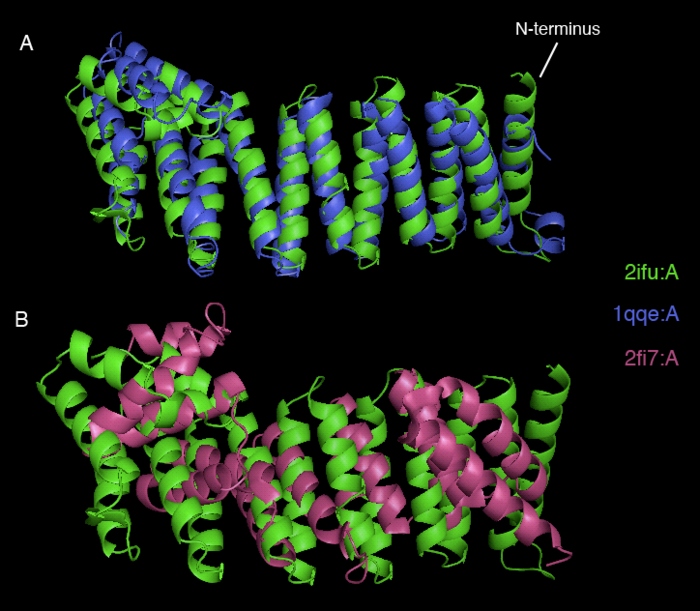
[[image:2ifu structure alognments.png|thumb|left|700px|[[Media:2ifu structure alognments.png|Figure 4. Superimposed structures of 2ifuA with 1qqeA (A) and 2ifuA with 2fi7A (B) generated by PyMOL. The 2ifuA is shown in green, 1qq3A in blue and 2fi7A in purple color.]]]] | [[image:2ifu structure alognments.png|thumb|left|none|700px|[[Media:2ifu structure alognments.png|Figure 4. Superimposed structures of 2ifuA with 1qqeA (A) and 2ifuA with 2fi7A (B) generated by PyMOL. The 2ifuA is shown in green, 1qq3A in blue and 2fi7A in purple color.]]]] | ||
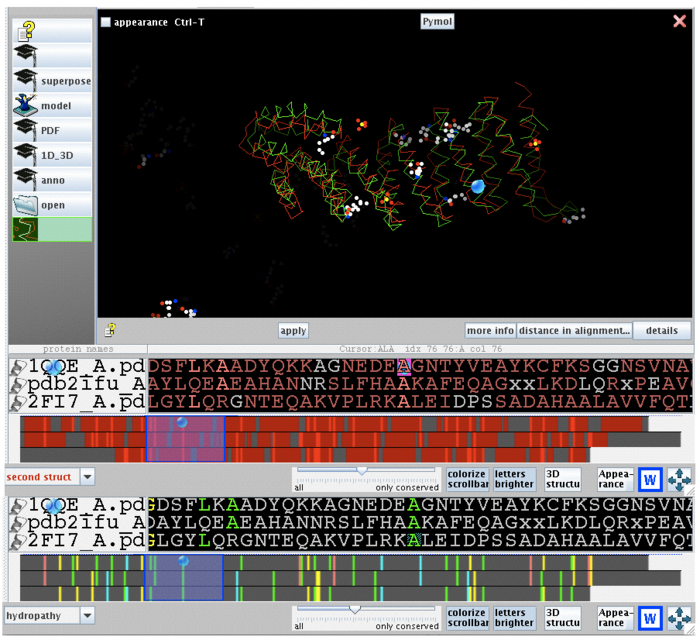
[[image:2ifu 1qqe 2fi7 alignment.png|thumb|left|700px|[[Media:2ifu 1qqe 2fi7 alignment.png|Figure 5. Stucture and sequence alignment of 2ifuA, 1qqeA and 2fi7A generated by STRAP. ]]]] | [[image:2ifu 1qqe 2fi7 alignment.png|thumb|left|none|700px|[[Media:2ifu 1qqe 2fi7 alignment.png|Figure 5. Stucture and sequence alignment of 2ifuA, 1qqeA and 2fi7A generated by STRAP. ]]]] | ||
==== physical properties ==== | ==== physical properties ==== | ||
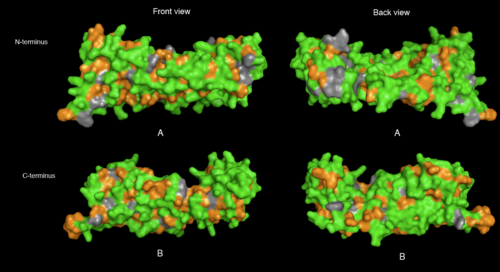
[[image:2ifuA-1qqe hydrophobes and hydrophilics.png|thumb|left|500px|[[Media:2ifuA-1qqe hydrophobes and hydrophilics.png|Figure 6. Hydrophobic and hydrophilic residues of 2ifuA (A) and 1qqeA (B) in front and back view. Amino acid residues with hydrophobic properties such as Ala, Gly, Val, Ile, Phe, and Met were selected and colored in green while that with hydrophilic properties (= Arg, Lys, His, Glu, Asp, Asn, Gln, Thr, Ser, and Cys) were colored in orange]]]] | [[image:2ifuA-1qqe hydrophobes and hydrophilics.png|thumb|left|none|500px|[[Media:2ifuA-1qqe hydrophobes and hydrophilics.png|Figure 6. Hydrophobic and hydrophilic residues of 2ifuA (A) and 1qqeA (B) in front and back view. Amino acid residues with hydrophobic properties such as Ala, Gly, Val, Ile, Phe, and Met were selected and colored in green while that with hydrophilic properties (= Arg, Lys, His, Glu, Asp, Asn, Gln, Thr, Ser, and Cys) were colored in orange]]]] | ||
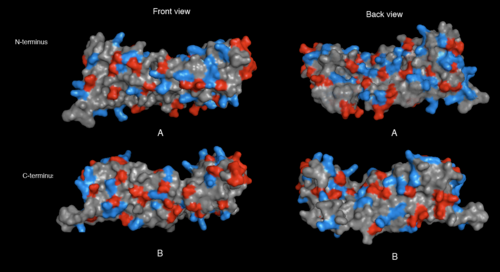
[[image:2ifuA-1qqe charged resi.png|thumb|left|500px|[[Media:2ifuA-1qqe charged resi.png|Figure 7. Charged residues of 2ifuA (A) and 1qqeA (B) in front and back view. The sidechains for charged residues indicated as red (negative; Glu and Asp) and blue (positive; Arg, Lys and His). ]]]] | [[image:2ifuA-1qqe charged resi.png|thumb|left|none|500px|[[Media:2ifuA-1qqe charged resi.png|Figure 7. Charged residues of 2ifuA (A) and 1qqeA (B) in front and back view. The sidechains for charged residues indicated as red (negative; Glu and Asp) and blue (positive; Arg, Lys and His). ]]]] | ||
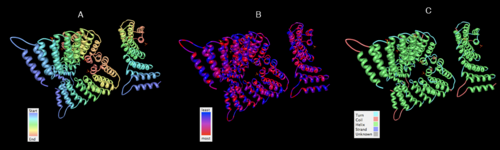
[[image:IfuA-structure.png|thumb|left|500px|[[Media:IfuA-structure.png|Figure 8. 2ifuA structures highlighted by chain color ramp (A), hydrophobicity (B) and conformation type (C). Legends are shown.]]]] | [[image:IfuA-structure.png|thumb|left|none|500px|[[Media:IfuA-structure.png|Figure 8. 2ifuA structures highlighted by chain color ramp (A), hydrophobicity (B) and conformation type (C). Legends are shown.]]]] | ||
[[image:Electrostatic potential (molecular surface).png|thumb|left|500px|[[Media:Electrostatic potential (molecular surface).png|Figure 9. 2ifuA electrostatic potential map visualised by molecular surface. Computation method that were used are coulumb method.](red= -1.800; white= 0.000; blue= +1.800]]] | [[image:Electrostatic potential (molecular surface).png|thumb|left|none|500px|[[Media:Electrostatic potential (molecular surface).png|Figure 9. 2ifuA electrostatic potential map visualised by molecular surface. Computation method that were used are coulumb method.](red= -1.800; white= 0.000; blue= +1.800]]] | ||
Figure 10 cleft | Figure 10 cleft | ||
Revision as of 11:25, 11 June 2007
SNAPG Structure
Structure architecture
In order to analyze protein structure of SNAPG, structural comparison to known protein structure is required. An insight to SNAPG structural arrangement provides various informative data on possible protein functions and interactions with another protein and/or DNA. Based on protein families database, Pfam at Sanger, it was found that SNAPG protein matched to Pfam-B protein families and consist of 2 domains, Pfam-B_7270 (PB007270) and Pfam-B_15198 (PB015189) respectively as shown in Figure 3. Both of 2 domains appears to be associated with NSF attachment protein activity (*dijelasin di discussion aja! NSF acivity dijelasin ga?)[1].
. The sequence was also used against InterproScan generated by Profunc that gave an TFR (Tetratricopeptide-like helical) domain classification while that of protein family agrees with Pfam classification.
(interproscan)
Structural comparison
Dali webserver is one of the powerful tool to screen any protein that are structurally homologous with our query. Two structurally related proteins with highest Z-value generated by Dali server were chosen for SNAPG structure comparison analysis. These proteins were vesicular transport ptotein sec17 (1qqe) and type 4 fimbrial biogenesis protein (2f17) (refer to Table 1).alto
| PDB-chain | Structure | Z-value | % identity | Protein |
|---|---|---|---|---|
| 2IFU-A | 37.8 | 100 | Endocytosis/exocytosis. Gamma-SNAP (Danio rerio) | |
| 1QQE-A | 23.3 | 23 | Protein binding. Vesicular transport protein sec17(yeast) | |
| 2FI7-A | 12.9 | 14 | Protein transport. Type 4 fimbrial biogenesis protein pili (Pseudomonas aeruginosa) |
2ifuA, 1qqeA and 2fi7 alignment
physical properties
[[image:Electrostatic potential (molecular surface).png|thumb|left|none|500px|Figure 9. 2ifuA electrostatic potential map visualised by molecular surface. Computation method that were used are coulumb method.](red= -1.800; white= 0.000; blue= +1.800]
Figure 10 cleft
SNAPG Function
Function by Similarity
SNAPG Evolution
Background
-> This is the cladogram treeview of SNAP-gamma protein. However, this view is not suitable enough to represent the evolutionary gap between species but this is clearly shows the species name.
Final Tree Analysis
-> Evolutionary analysis of the tree with radial view (for professional viewer)
Legends:
- Red-dotted sign means boostrap value more than 75.00 or 75%
- any number (in red) located near branching tree indicated boostrap value of the branching tree
- Green Line: The species belongs to Plants group
- Brown Line: Amoeba group
- Purple Line: Parasite type
- Pink Line : The species belongs to Insects Group
- Light Green Line: Worms Group
- Dark Blue Line: Invertebrate
- Red Line: Vertebrate
Other version: Evolutionary analysis of the tree with radial view (for non-professional or common viewer)
Other Attributes
List IDs use as an batch entrez input