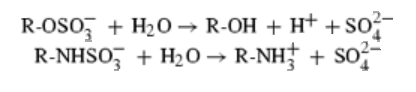3b5q Structure and Function
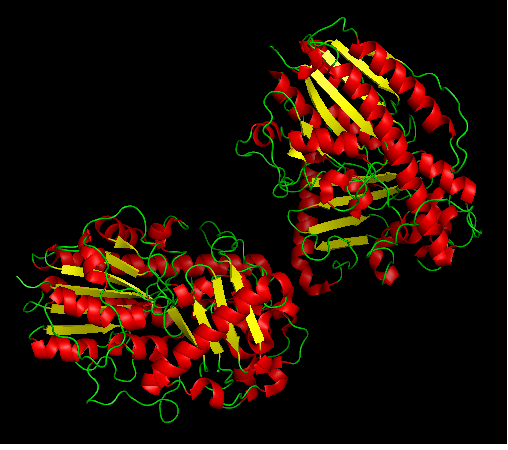
Arylsulfatase K structure
Protein data bank profile characteris arylsulfatase as a hydrolase and a sulfatase. A Sulfatase hydrolyses sulfate esters bonds of substrates, including O-sulfates and N-sulfates as shown below.
Sulfatases are enzymes,which hydrolyse sulfate ester bonds of substrates. These are categorised as EC 3.1.6. in enzyme classifications. Most of the family members has shown to contain a highly conserved cystine residue and a bivalent metal binding site.
Arylsulphatase A (ASA) is a lysosomal enzyme which hydrolyzes cerebroside sulphate. Arylsulphatase B (ASB),also a lysosomal enzyme, which hydrolyzes the sulphate ester group from N-acetylgalactosamine 4-sulphate group of dermatan sulphate.
Sequence conservation
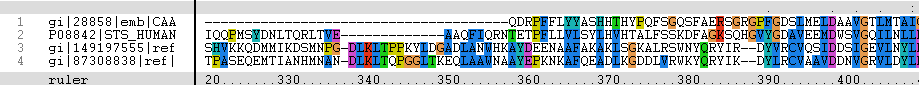
MSA data revealed several highly conserved residues in the N-terminal region of all sequences aligned. These residues are shown below as an alignment of Arylsulfatase K with the sequence of human stroid sulfatase.
Figure 1: Multiple sequence alignment (MSA). Residues which are concerved across the entire sulfatase family are marked in red
Structural alignment
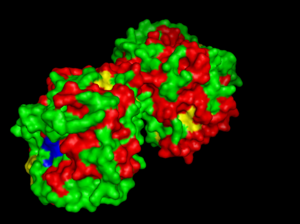
- Three dimentional structure of arylsulfatase was aligned with other available structures using DALI server (structural alignment). Results are shown in 'figure 4'.
No: Chain Z rmsd lali nres %id Description 1: 3b5q-A 73.6 0.0 464 464 100 MOLECULE: PUTATIVE SULFATASE YIDJ; 2: 3b5q-B 70.2 0.3 464 467 100 MOLECULE: PUTATIVE SULFATASE YIDJ; 3: 2qzu-A 35.1 2.5 375 465 25 MOLECULE: PUTATIVE SULFATASE YIDJ; 4: 1fsu 28.7 2.8 344 474 22 MOLECULE: N-ACETYLGALACTOSAMINE-4-SULFATASE; 5: 1n2l-A 28.4 3.0 343 483 25 MOLECULE: ARYLSULFATASE A; 6: 1n2k-A 28.1 3.1 342 482 25 MOLECULE: ARYLSULFATASE A; 7: 1e2s-P 28.1 3.0 341 481 26 MOLECULE: ARYLSULFATASE A; 8: 1e3c-P 28.0 3.1 341 481 26 MOLECULE: ARYLSULFATASE A; 9: 1e33-P 28.0 3.1 342 480 25 MOLECULE: ARYLSULFATASE A; 10: 1e1z-P 27.9 3.0 341 481 26 MOLECULE: ARYLSULFATASE A; 11: 1auk 27.8 3.1 340 481 26 MOLECULE: ARYLSULFATASE A; 12: 1p49-A 27.7 2.9 338 548 24 MOLECULE: STERYL-SULFATASE; 13: 1hdh-B 27.4 3.1 365 525 23 MOLECULE: ARYLSULFATASE; 14: 1hdh-A 27.4 3.0 363 525 23 MOLECULE: ARYLSULFATASE; 15: 2rh6-A 24.3 2.6 257 382 14 MOLECULE: PHOSPHODIESTERASE-NUCLEOTIDE PYROPHOSPHATASE; 16: 2rh6-B 24.2 2.6 257 382 14 MOLECULE: PHOSPHODIESTERASE-NUCLEOTIDE PYROPHOSPHATASE;
Figure 4: Structurally related proteins. (No 1 and 2 are two chains of arylsulfatase K).
- The function of highly related proteins were found using ProFunc
- Arylsulfatase A has both sulfuric ester hydrolase and phosphoric monoester hydrolase activities and localised in lysosomes.
- Steryl-sulfatase is a sulfuric ester hydrolase found in endoplasmin reticulums.
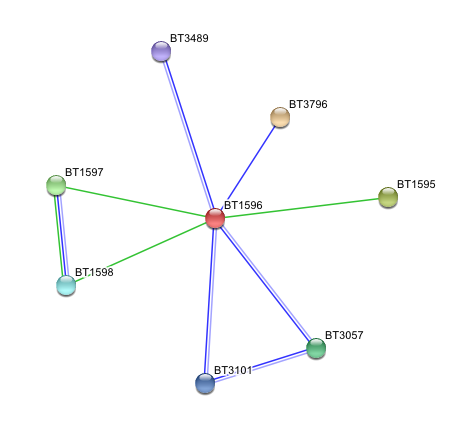
Arylsulfatase K (BT1596) Interactions with other proteins
Input Protein
- BT1596 Putative sulfatase yidJ (481 aa) of Bacteroides thetaiotaomicron.
Predicted Functional Partners
- BT3796: Putative secreted sulfatase ydeN (518 aa).
- BT1595 Transcription termination factor rho (722 aa).
- BT1597 Two-component system sensor histidine kinase (539 aa).
- BT3057 N-acetylgalactosamine-6-sulfatase (508 aa).
- BT1598 Putative two-component system sensor histidine (655 aa).
- BT3101 N-sulphoglucosamine sulphohydrolase (455 aa).
- BT3489 Arylsulfatase B {UniProtKB/TrEMBL-Q8A219} (458 aa).
N-acetylgalactosamine-6-sulfatase cleaves the 6-sulfate groups of N-acetyl-D-galactosamine 6-sulfate units in chondroitin sulfate and D-galactose 6-sulfate units in keratan sulfate. N-sulphoglucosamine sulphohydrolase is also known as heparine sulfamidase, which catalyses the hydrolysis of Sulfur-Nitrogen bonds. N-sulphoglucosamine sulphohydrolase is responsible for the degradation of glucosaminlglycan and glycan structure of extra cellular matrix.
- N-sulfo-D-glucosamine + H(2)O <=> D-glucosamine + sulfate
N-acetylgalactosamine-4- sulfatase (Arylsulfatase B) hydrolyse the sulfate ester group from N-acetylgalactosamine 4-sulfate of dermatine sulfate. Deficiency of ASB cause a rare mucopolysaccharidosis (MPS IV; Maroteaux-Lamy syndrome)
All these enzymes has generaly the same function, but acts on different substrate.
Finding the functional site and mechamism of action
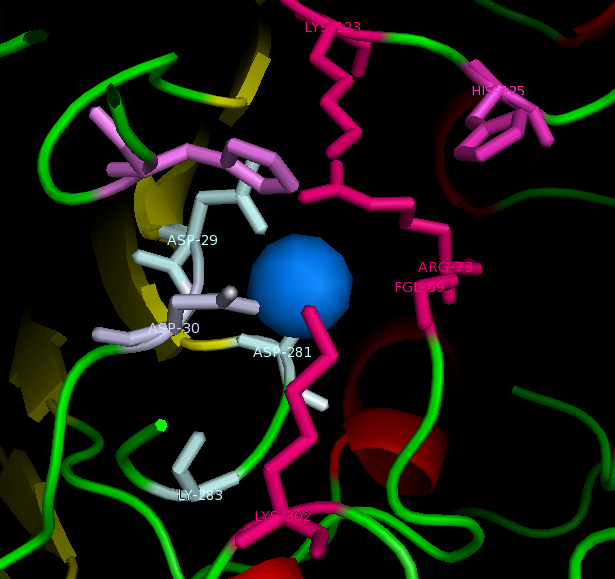
Published data on catalytic activity of STS and ASA was found in literature search. Both STS and ASA shows a divalent metal binding site in the catalytic site, but th crystal structure of ASK doesn't show this. However, three out of four residues which provide oxygen ligans to hold the cation are conserved in ASK. They are shown highlighted in purple in STS-ASK alignment.
The Aspartic acid (D) in 35th position of STS is immidiatly followed by a second D, which is substituted by Histidine (H) in ASK. But when the site chain organisation of two residues are examined, it is a possibility for one Nitrogen in five-member-ring to donate a lone pair to form a coordinate bond with a divalent cation. Therefore ASK active site may contain a divalent cation in biologycal situations.
Three out of five catalytic resideus were also conserved between ASK and STS ( shown highlighted in red). The characteristic Cystine residue of nearly all sulfatases in conserved in STS, but substituted by Serine (S) in ASK. Biologycally acive STS has C post translationally modified in to a Formylglycine (FG), and subsequiently to a Hydroxyformylglycine (HFG) during the reaction. The crystal structure doesn't show a FG in ASK structure, however this may be a result of inadequite resolution. The crystal structure has obtained at 2.4 A resolution while bond lenghts of C=O and C-O are 1.2 A and 1.4 A respectively. Further evidence from literature suggests that a subset of ASK found in bacteria including Bacteroides thetaiotamicron and Klebsiella pneumoneae use a S, post translationally modified to a FG, for the same cataltic action[[1]].Finally H290 of STS in not conserven in either ASK or ASA, but the 3D structure shows a different H in the colse proximity and this H in ASA is conserved in most ASA from different species.
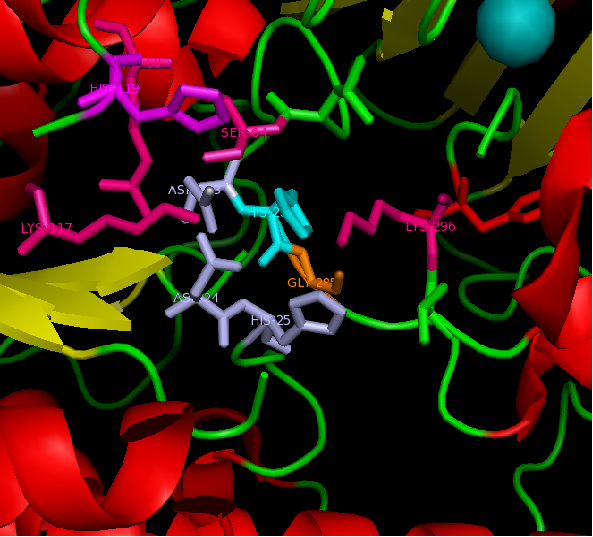
Conservation of the C-terminus
C-terminus of sulfatases are recognised to contain a substrate binding site, hence weakly conserved throughout the family due to the variation of types of substrates used. However, when the C-terminal regions in two sequence alignment of ASK and STN was looked at, the level of conservation was very high. Based on this evidence it is predicted, that STS and ASK shair substrates.
Possible function and likely substrates of ASK
STS is a membrane bound enzyme, mostly found in human placenta and skin fibroblasts. It converts sex-stroid precursors to ative estrogen and androgen, thereofre give a local suppley of these hormones (Ghosh, D., 2007). ASK is a water soluble enzyme localysed in ER. No tissue localization is specified to the data. Based on all the above evidence, the possible function of ASK may be binding and activating stroids in the ER lumen.
Click here to go Back