ATP binding domain 4 Functions
ATP pyrophosphatase activity commonly have a widespread motif of ([A/S]-[F/y]-S-G-G-[L/V]-D-T-[S/T] which is commonly conserved in many groups of enzymes including GMP synthetases, argininosuccinate synthetases and ATP sulfurylases. (Lemke et al. 2001) If this PP-motif also presents in our uncharacterised protein, this may give up a clue to its functions.
Consensus of alignment between our sequence and other ATP pyrophosphatase members demonstrates that this protein belongs to anabolic pathways and have ATP pyrophosphatase activity, meaning that they will participate in hydrolysing the Alpha-Beta bond in ATP. Moreover, the respective overall reaction schemes are different among different family members.
For examples, according to the table below, in the reaction scheme of argininosuccinate synthetase (Assy), the cleavage of ATP will provide energy for changing L-citrullin and L-Asp into L-argininosuccinate. Whereas, in ATP sulfurylase, ATP bond breaking will drive the sulfate transfer to AMP. Therefore, although our protein may involve in ATP high energy bond cleavage, we are not sure what type of subunit will our protein associate with the energy-requiring function since the reaction schemes of different proteins are different.
Structural-based methods:Matching folds by sequence comparison (SSM)
The ProFunc server is used to identify similarities in structure and hopefully can indicate conserved functions. The SSM (Secondary Structure Matching) program is used to compare the fold of our query protein (Putative n-type ATP pyrophosphatase) against the folds of the structural database in PDB. The secondary structure elements (SSEs) of two proteins are compared and ranked by Z-scores, which is computed from the matching value obtained from structural superpositions.
The fold match results for our query protein (1ru8 chain A) returns with 10 structural matches.
The top match (1ru8A) is our query sequence and so does the second match. The 3rd hit (2d13A) is a hypothetical dimeric protein from a related species, Pyrococcus horikoshii expressed from E. Coli. The result returns with a high value of 15 matched secondary structure elements. (as shown by No.SSE column) These two proteins have a high similarities in terms of helices and strands. This result is also coherent with Dali search too. However, the function of this protein is also unidentified and therefore of little use to us.
The hits beyond 3, colour-coded with orange and blue, are rather weak matches and unlike hits; which can also be visualised from the Dali search (Krissinel & Henrick 2004) Few matches are shown in the alignment view.
Nests analysis
Nests are structural motifs that are particularly important for its functions. These nests are usually hidden deep within clefts to stay away from hydrophilic environment. These structural motifs are usually parts of hydrogen bond and may form charge-charge interactions with anionic groups or ligands. Nests are identified by computer programmes based on their alternative enantiomeric mainchain dihedral angles from the alpha and gamma regions of a Ramachandran plot. The results are shown as follows.
High nest scores of nests 1-3 suggests that the first 3 sites are accessible to solvent, having significant conservative score compared to their parent residues or associated with those larger surface clefts. First 3 scores are all above 2.0 which are subjective of nests being relatively functionally significant.
Non-zero solvent accessibility: the percentage solvent accessible surface area of the residue's main chain nitrogen atom. As the first 3 residue ranges are non-zero then these atoms are accessible to solvent and capable of interacting with a binding anion, Leu4(A) in particular is very accessible to solvent.
Deeply clefted of nest 1 gives a strong indication of how deep in the cleft the NH atoms lie. As Ser12(A) and Gly14(A) lie particularly deep in the largest cleft 1, it is a strong indicator that they are functionally important.
Highly residue conserved in nests 1 and 2 has been determined from a multiple sequence alignment of the protein's sequence against BLAST hits from the UniProt sequence database. The conservation score of 1.0 in nests 1 and 2 indicates they are perfectly conserved.
1. Watson JD and Milner-White EJ (2002). A novel main-chain anion-binding site in proteins: the nest. A particular combination of phi,psi values in successive residues gives rise to anion-binding sites that occur commonly and are found often at functionally important regions. J. Mol. Biol., 315, 171-82. 2. Watson JD and Milner-White EJ (2002). The conformations of polypeptide chains where the main-chain parts of successive residues are enantiomeric. Their occurrence in cation and anion-binding regions of proteins. J. Mol. Biol., 315, 183-191. 3. Pal D, Suhnel J and Weiss MS (2002). New principles of protein structure: nests, eggs - and what next? Angew. Chem. Int. Ed., 41, 4663-4665.
Cleft analysis
The following result table demonstrates the gaps in the order of decreasing volumes. Clefts and cavities in our protein have been colour-coded according to the table below. In gap region 1, this biggest cleft contains 42 residue conservations (shown in red letters in ‘Residue conservation’ column. A TRS which is a common crystallization solvent ligand was shown to bind to gap 1.
Krissinel, E and henrick, K. (2004), Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions, Acta Cryst. D60: 2256-2268.
Lemke, C, T. and Howell, P.L. (2001), The 1.6A Crystal Structure of E.coli Argininosuccinate Synthetase Suggests a Confomational Change during Catalysis, Structure 9:1153-1164.
My sequences:Putative n-type ATP pyrophosphatase from Pyrococcus furiosus, the Northeast Structural Genomics Target PfR23
AAHN-like superfamily
Adenine nucleotide alpha hydrolases, AANH_like (cl00292), superfamily includes members of N type ATP PPases, ATP sulphurylases Universal Stress Response protein and electron transfer flavoprotein (ETF). The domain forms a alpha/beta/alpha fold which binds to Adenosine nucleotide. Our sequence of interest is a putative n-type ATP pyrophosphatase that are under AAHN-like superfamily.
Pfam from Sanger suggests that N-type ATP Pyrophosphatase belongs to a family named ATP-binding 4 (PF01902), which contains a 200 amino acids long strongly conserved motif of SGGKD near the N-terminus.
The family ATP-binding 4 is a member of clan PP-loop (CL0039), which is comprised of 9 members: Arginossucinate synthase, Asn synthase, ATP binding 3, Exs B, NAD synthase, PAPS reductase, Thianmine biosynthesis protein, tRNA Methyl transferase and finally where our sequence belongs to -- ATP binding 4.
Although our protein sequence shows little homology with human ATP Binding domain 4 proteins, having % sequence ID = 36.8%, understanding the function of human ATPPD4 can help us infer functions of our sequence.
Dali analysis of Domain Alignment
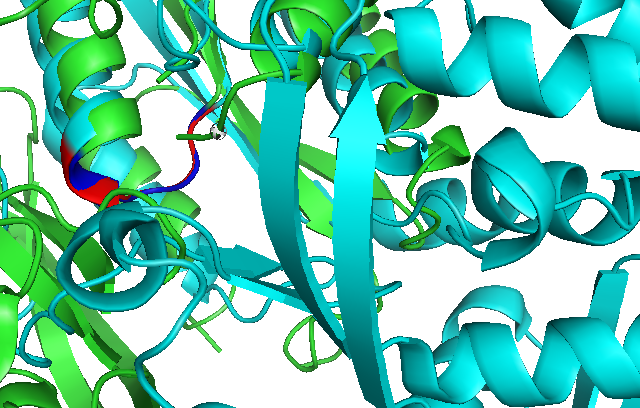
Use of Dali reveals similar domain alignment of our sequence with Argininosuccinate Synthase (2nz2-A), which Z-score is 11.0% (indicatively significant domain similarity). Therefore, we use Pymol to align conserved residues of 1RU8 and 2NZ2 (align 1RU8 & i. 11-16, 2NZ2 & i. 11-15). Close structure resemblance is a strong indication of function resemblance.
Profunc Secondary Structural Matches
Profunc Nest analysis for structural motifs
Nest analysis results for our sequence (chain A). Nests are structural motifs that are often found in functionally important regions of the protein structures. 7 nests have been located in our chain A.
High nest scores of nests 1-3 suggests that the first 3 sites are accessible to solvent, having significant conservative score compared to their parent residues or associated with those larger surface clefts. First 3 scores are all above 2.0 which are subjective of nests being relatively functionally significant.
Non-zero solvent accessibility: the percentage solvent accessible surface area of the residue's main chain nitrogen atom. As the first 3 residue ranges are non-zero then these atoms are accessible to solvent and capable of interacting with a binding anion, Leu4(A) in particular is very accessible to solvent.
Deeply clefted of nest 1 gives a strong indication of how deep in the cleft the NH atoms lie. As Ser12(A) and Gly14(A) lie particularly deep in the largest cleft 1, it is a strong indicator that they are functionally important.
Highly residue conserved in nests 1 and 2 has been determined from a multiple sequence alignment of the protein's sequence against BLAST hits from the UniProt sequence database. The conservation score of 1.0 in nests 1 and 2 indicates they are perfectly conserved.
1. Watson JD and Milner-White EJ (2002). A novel main-chain anion-binding site in proteins: the nest. A particular combination of phi,psi values in successive residues gives rise to anion-binding sites that occur commonly and are found often at functionally important regions. J. Mol. Biol., 315, 171-82.
2. Watson JD and Milner-White EJ (2002). The conformations of polypeptide chains where the main-chain parts of successive residues are enantiomeric. Their occurrence in cation and anion-binding regions of proteins. J. Mol. Biol., 315, 183-191.
3. Pal D, Suhnel J and Weiss MS (2002). New principles of protein structure: nests, eggs - and what next? Angew. Chem. Int. Ed., 41, 4663-4665.
String Functional Partners Predictions
Literature
- ATP pyrophosphatase (ATP PPase) is used to assist lysidine formation using a lysine-specific loop and tRNA recognition domain
- Lysidine is a lysine-combined modified cytidine, locating at antcodon wobble position (34) of bacterial tRNA
- Usually ATP-pyrophosphatatse has a domain of GMP synthetase, for adding adenine and help lysine attack on Carbon atom.
http://www.pnas.org/content/102/21/7487.abstract
--
- NAD+ synthetase belongs to a member of the family of N-type ATP pyrophosphatase (ATP PPases)
- Some other members of N-type ATP pyrophosphatase include NAD+ synthetase, GMP synthetase, asparagine synthetase and argininosuccinate synthetase
- this family is characterised by strictly conserved fingerprint sequence Ser-Gly-Gly-X-Ser/ Thr-Ser/ Thr at P-loop (this is found by the comparison of 3-D structures of NAD+ synthetase and GMP synthetase
- Since they are in the same family, we can infer their structure similarities and propose the functions of our sequences. - Look for the ATP-binding sites on ref[11]
- Rizzi, M., et al., & Galizzi, A. (1996). Crystal structure of NH3-dependent NAD synthetase from Bacillus subtilis. EMBO J. 15, 5125-5134
--
- ([A/S]-[F/y]-S-G-G-[L/V]-D-T-[S/T] is a common consensus sequences that contains a glycine-rich motif that is common to a subset of ATP pyrophosphatases, which is known as 'N-type' ATP pyrophosphatases.
- N-type ATP pyrophosphatases all catalyses a substrate adenylation to activate a carbonyl C=O or carboxyl COO- group for the subsequent attack of a nitrogen nucleophile.
- This glycine-rich motif will form a modified P loop within the nucleotide binding domain. Additionally, the classical P loops of ATP-dependent proteins will interact with the single terminal phosphate of nucleotide, and then this P-loop binds with pyrophosphate moiety, and therefore this sequence is known as 'PP-motif'.
- Lemke, C, T. and Howell, P.L. (2001), The 1.6A Crystal Structure of E.coli Argininosuccinate Synthetase Suggests a Confomational Change during Catalysis, Structure 9:1153-1164.
--
Taxonomic distribution of Adenine nucleotide alpha hydrolases-like domains in all kingdoms.
Each node represents the features of a single taxonomic group, or organism. The nodes are arranged hierarchically in concentric rings. The parent taxon, located in the centre, leads recursively outwards towards its children. The size of the circle indicates the mean number of domains found per organism in a given taxonomic group. For individual organisms, it gives the actual number of domains.
Gough, J., Karplus, K., Hughey, R. and Chothia, C. (2001). "Assignment of Homology to Genome Sequences using a Library of Hidden Markov Models that Represent all Proteins of Known Structure." J. Mol. Biol., 313(4), 903-919.