ATP binding domain 4 Structures: Difference between revisions
Anharmustafa (talk | contribs) No edit summary |
Anharmustafa (talk | contribs) |
||
| Line 45: | Line 45: | ||
[[Image:PDBsum11.PNG|framed|left|500px]] | [[Image:PDBsum11.PNG|framed|left|500px]] | ||
Revision as of 15:50, 1 June 2009
General information
General information from PDB indicates that :
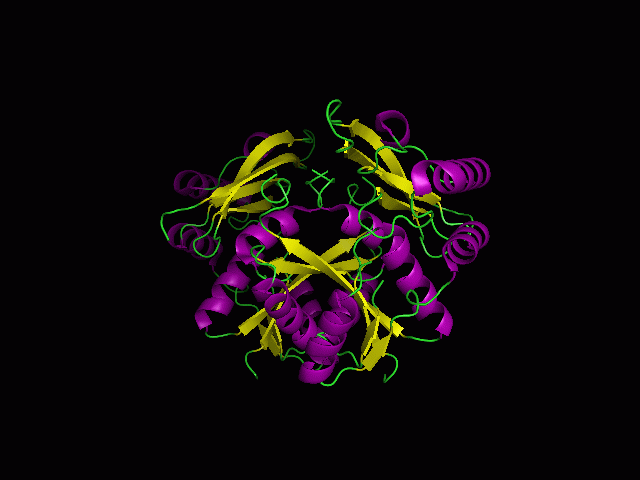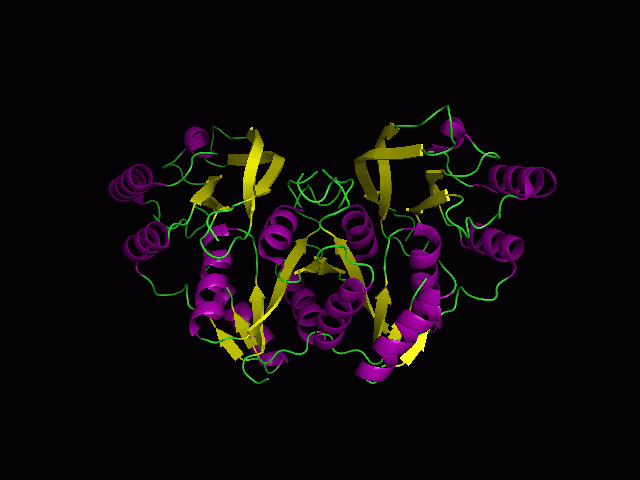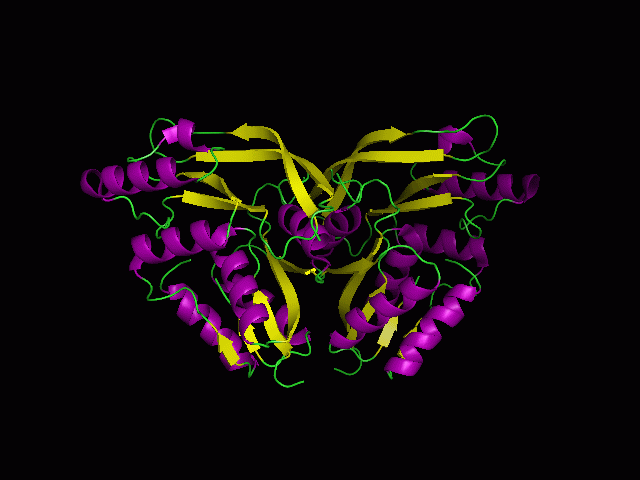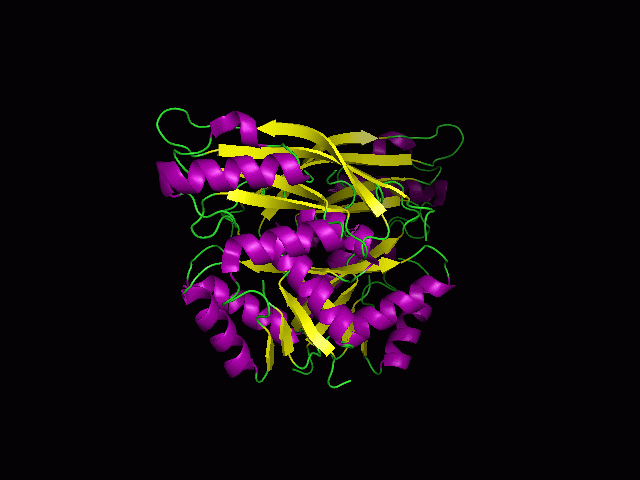
(a) 1RU8 is a putative n-type ATP pyrophosphatase isolated from Pyrococcus furiosus, expressed in Escherichia Coli.
(b) Is a member of clan PP-loop
(c) Resolution of 2.7 angstroms, with an r-value of 0.218.
(d) Ligand chemical component identified as TRS (2-AMINO-2-HYDROXYMETHYL-PROPANE-1,3-DIOL).
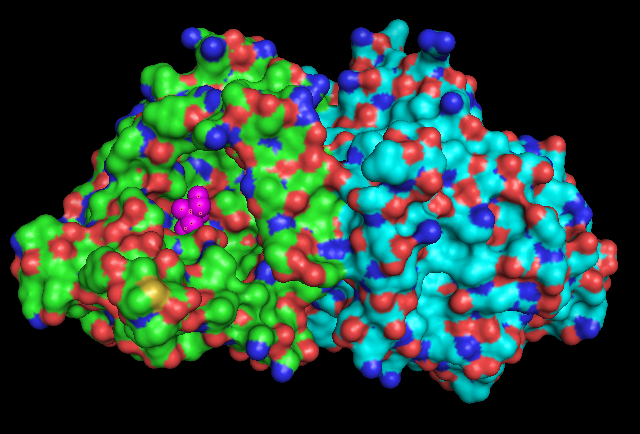
Surface Structure
Pymol Visualization
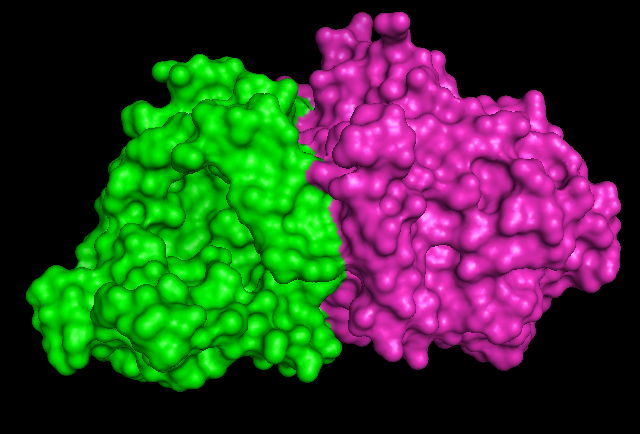
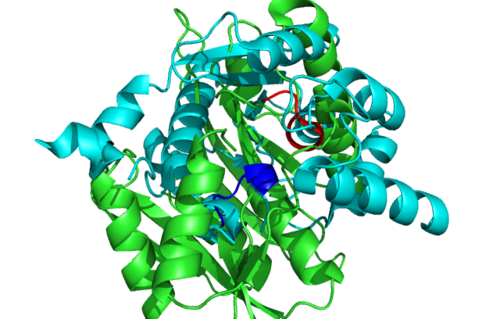
1RU8 is a dimer
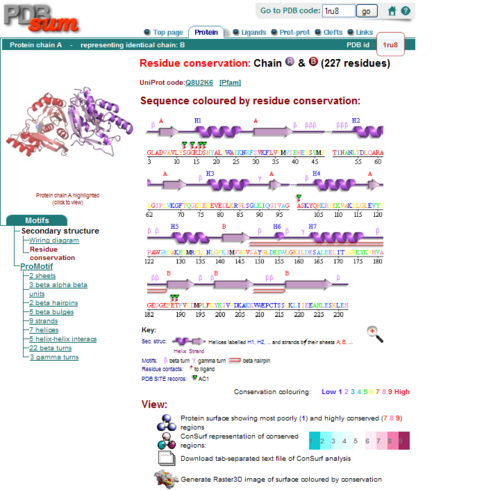
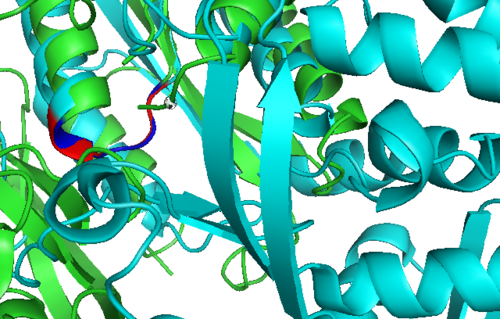
Secondary Structure and Location of PP-loop
Potential Ligand Binding Interaction
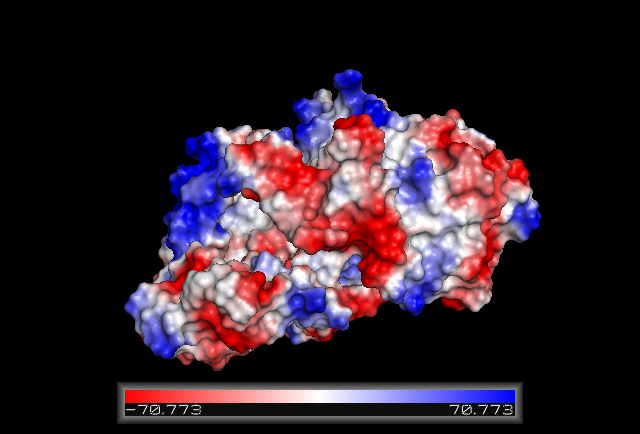
Electrostatic Surface Potential
Structure Similarities
Z score , the statistical significance of the similarity between protein-of-interest and other neighbourhood proteins. The program optimizes a weighted sum of similarities of intramolecular distances.
Root Mean Square Distance (RMSD), root-mean-square deviation of C-alpha atoms in the least-squares superimposition of the structurally equivalent C-alpha atoms. RMSD is not optimized and is only reported for information.
lali, the number of structurally equivalent residues.
nres, or the total number of amino acids in the hit protein.
%id, percentage of identical amino acids over structurally equivalent residues.
Structure Alignment
1RU8 and 2NZ2
1RU8 and 3BL5
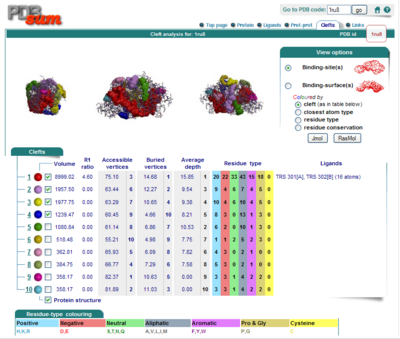
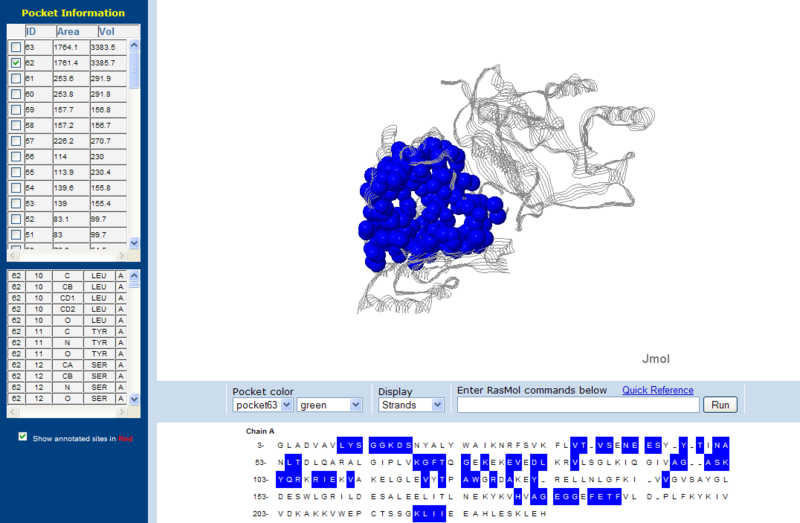
Surface Clefts
Surface Topography