ATP binding domain 4 Structures: Difference between revisions
Anharmustafa (talk | contribs) No edit summary |
Anharmustafa (talk | contribs) |
||
| Line 74: | Line 74: | ||
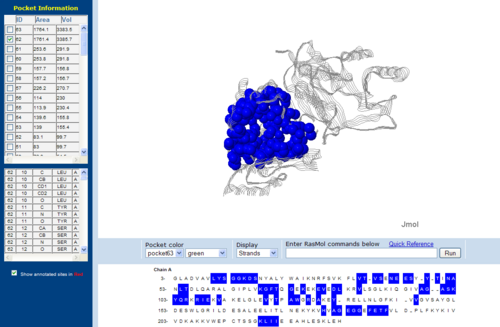
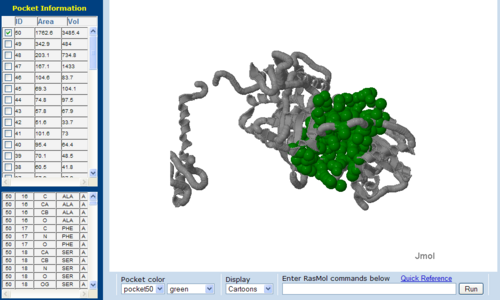
[[Image:CAST-2nz2.PNG|right|thumb|500px|'''Figure 13'''. The topology of 2NZ2 generated via CASTp]] | [[Image:CAST-2nz2.PNG|right|thumb|500px|'''Figure 13'''. The topology of 2NZ2 generated via CASTp]] | ||
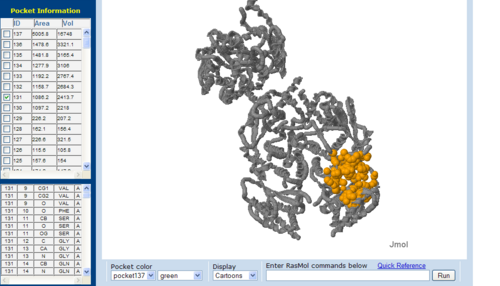
[[Image:CAST-3bl5.PNG|left|thumb|500px|'''Figure 14'''. The topology of 3BL5 generated via CASTp]] | [[Image:CAST-3bl5.PNG|left|thumb|500px|'''Figure 14'''. The topology of 3BL5 generated via CASTp]] | ||
<BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> | |||
The volume and size of the cleft of between protein indicated similarity between 1RU8 and 2NZ2. While 3BL5 less similarity when compared to both 1RU8 and 2NZ2 | |||
Revision as of 12:54, 2 June 2009
General Properties
General information from PDB indicates that :
(a) 1RU8 is a putative n-type ATP pyrophosphatase isolated from Pyrococcus furiosus, expressed in Escherichia Coli.
(b) Is a member of clan PP-loop
(c) Resolution of 2.7 angstroms, with an r-value of 0.218.
(d) Ligand chemical component identified as TRS (2-AMINO-2-HYDROXYMETHYL-PROPANE-1,3-DIOL).
Surface Structure
Pymol Visualization
Secondary Structure and Location of PP-loop
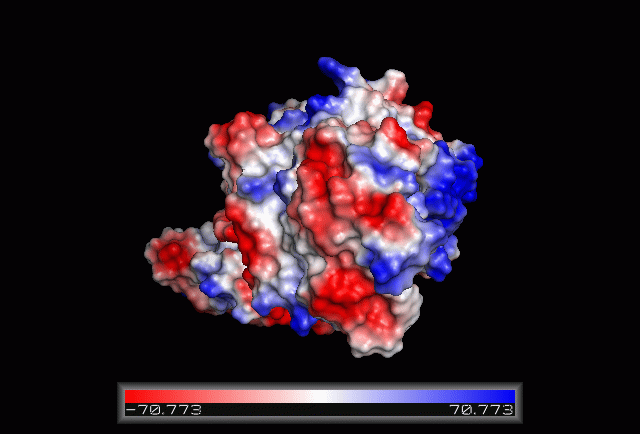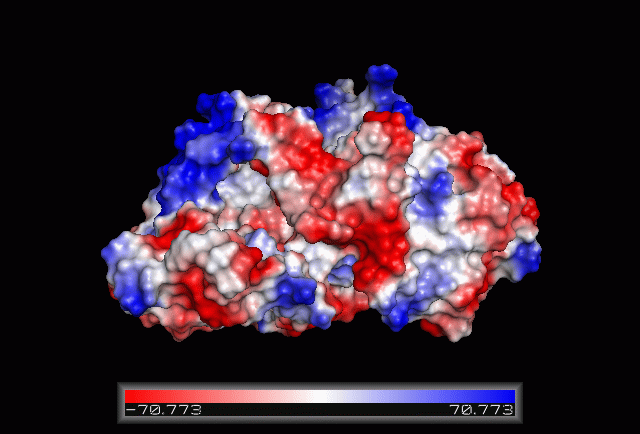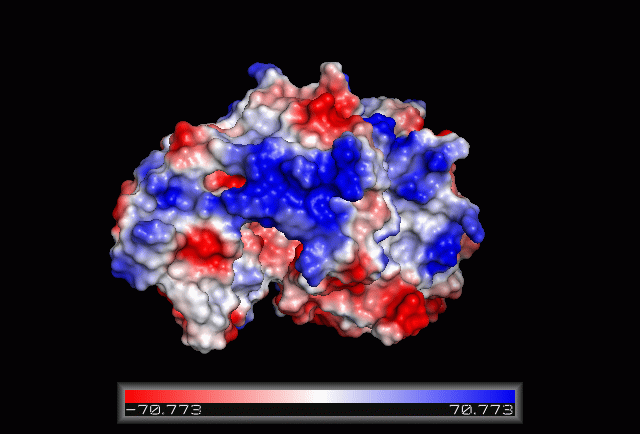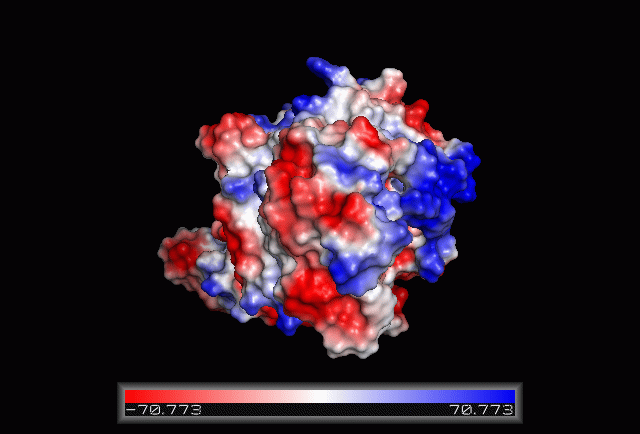
Electrostatic Surface Potential
Structure Similarities
Z score , the statistical significance of the similarity between protein-of-interest and other neighbourhood proteins. The program optimizes a weighted sum of similarities of intramolecular distances.
Root Mean Square Distance (RMSD), root-mean-square deviation of C-alpha atoms in the least-squares superimposition of the structurally equivalent C-alpha atoms. RMSD is not optimized and is only reported for information.
lali, the number of structurally equivalent residues.
nres, or the total number of amino acids in the hit protein.
%id, percentage of identical amino acids over structurally equivalent residues.
Structure Alignment
1RU8 and 2NZ2
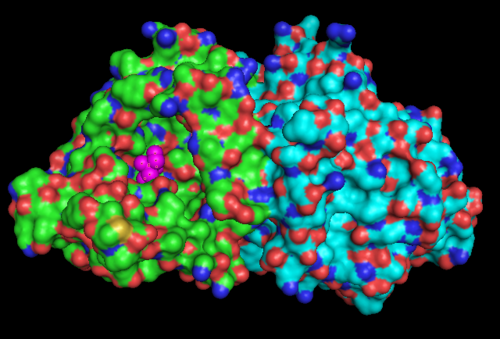
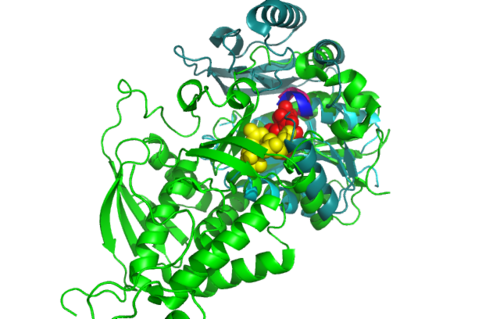
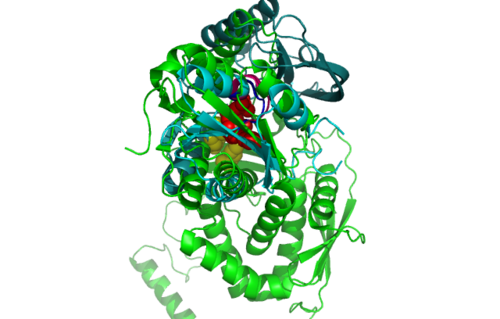
Highlighted in green is the 2NZ2 while in magenta is the 1RU8. Since 1RU8 has 2 domain, domain B is coloured darker in magenta for differentiation. There are 2 ligand indicated by the yellow (Citrulline) and red (ATP) spheres from 2NZ2. Highlighted also is the conserved region of PP-loop in pink and blue for 1RU8 and 2NZ2 respectively.
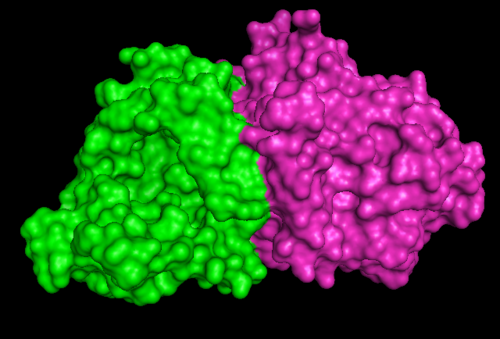
1RU8 and 3BL5
Surface Cleft and Topography
The volume and size of the cleft of between protein indicated similarity between 1RU8 and 2NZ2. While 3BL5 less similarity when compared to both 1RU8 and 2NZ2