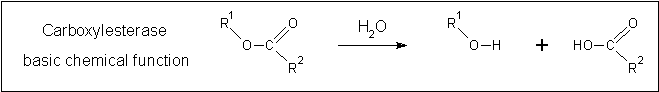Arylformamidase Discussion: Difference between revisions
Thomasparker (talk | contribs) No edit summary |
Thomasparker (talk | contribs) No edit summary |
||
| Line 8: | Line 8: | ||
The HSL family encompasses a broad range of proteins with diverse functionality. Even though a number of HSL proteins have been determined and were included in the structure search, 2c7b was still returned as the most similar structure, indicating that 2pbl may in fact be a carboxylesterase. Specifically, carboxylesterases are involved in a broad range of reactions. Fundamentally, they catalyze the hydration of carboxyl esters into an alcohol and a carboxylic acid (see …). | The HSL family encompasses a broad range of proteins with diverse functionality. Even though a number of HSL proteins have been determined and were included in the structure search, 2c7b was still returned as the most similar structure, indicating that 2pbl may in fact be a carboxylesterase. Specifically, carboxylesterases are involved in a broad range of reactions. Fundamentally, they catalyze the hydration of carboxyl esters into an alcohol and a carboxylic acid (see …). | ||
[[Image:carboxylesterase_reaction.png|Framed|Centre|'''Figure ...:''' ''Fundamental reaction mechanism of carboxylesterases.'']] | |||
Further analysis revealed that 2pbl shared greater structural similarity with thermostable esterases of the HSL family compared to its other members. As discussed, thermostable proteins such as 2c7b often exist in dimers, a possiblity which remains uninvestigated for 2pbl. | Further analysis revealed that 2pbl shared greater structural similarity with thermostable esterases of the HSL family compared to its other members. As discussed, thermostable proteins such as 2c7b often exist in dimers, a possiblity which remains uninvestigated for 2pbl. | ||
Revision as of 09:12, 3 June 2008
probable function
Catalytic triad found in the canonical positions observed in a wide variety of α/β hydrolases.
Functional inference from sequence similarity was restricted due to lack of supporting literature for the highest-scoring sequences. Although the function of the arylformamidase from Mus musculus has been relatively well-characterised, its structure has not been determined. Whilst a high-degree of conservation of the catalytic triad has been observed, there are structurally more-similarly proteins with an even higher degree of conservation.
The carboxylesterase 2c7b was chosen for further analysis due to its high structural similarity to 2pbl. 2c7b is a thermostable carboxylesterase from an uncultured archaeon which was isolated in a thermal environmental sample (Byun 2007). This carboxylesterase belongs to a family of bacterial hormone-sensitive lipases/esterases (HSLs) known for their amino-acid sequence similarity to the mammalian hormone-sensitive lipase (ARPIGNY 1999). There were a number of other bacterial HSL proteins found to have a similar structure to 2pbl. Although in all cases there was a relatively low sequence similarity with 2pbl, regions characteristic to HSL proteins were found to be conserved indicating that 2pbl may indeed be another bacterial HSL (Arpigny 1999, see figure…).
The HSL family encompasses a broad range of proteins with diverse functionality. Even though a number of HSL proteins have been determined and were included in the structure search, 2c7b was still returned as the most similar structure, indicating that 2pbl may in fact be a carboxylesterase. Specifically, carboxylesterases are involved in a broad range of reactions. Fundamentally, they catalyze the hydration of carboxyl esters into an alcohol and a carboxylic acid (see …).
Further analysis revealed that 2pbl shared greater structural similarity with thermostable esterases of the HSL family compared to its other members. As discussed, thermostable proteins such as 2c7b often exist in dimers, a possiblity which remains uninvestigated for 2pbl.
evolutionary link...
biological implications/applications...
Potential thermostability - applicability in industry.
further research...