Arylformamidase Function: Difference between revisions
From MDWiki
Jump to navigationJump to search
Thomasparker (talk | contribs) |
Thomasparker (talk | contribs) |
||
| Line 8: | Line 8: | ||
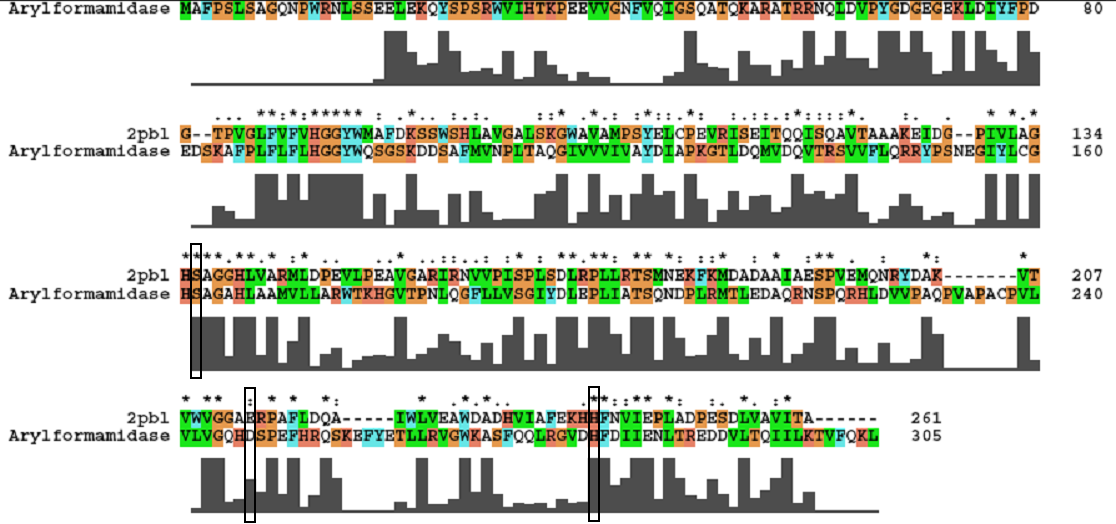
A review of the DALI output revealed many structures similar to 2pbl with little sequence identity. The most similar of these, 2cb7, is a thermophilic and thermostable carboxylesterase. The catalytic triad responsible for its activity has been identified, and in determining whether 2pbl shared similar functionality, conservation of the catalytic triad between the two proteins was assessed (see figure 1). | A review of the DALI output revealed many structures similar to 2pbl with little sequence identity. The most similar of these, 2cb7, is a thermophilic and thermostable carboxylesterase. The catalytic triad responsible for its activity has been identified, and in determining whether 2pbl shared similar functionality, conservation of the catalytic triad between the two proteins was assessed (see figure 1). | ||
[[Image:2cb7alignment.jpg]] | [[Image:2cb7alignment.jpg]] | ||
Figure 1: | '''''Figure 1:''' Conservation of the ... catalytic triad between 2cb7 and 2pbl.'' | ||
== Evidence from Similar Sequences == | == Evidence from Similar Sequences == | ||
Revision as of 09:13, 27 May 2008
Lines of evidence:
Evidence from Similar Structures
A review of the DALI output revealed many structures similar to 2pbl with little sequence identity. The most similar of these, 2cb7, is a thermophilic and thermostable carboxylesterase. The catalytic triad responsible for its activity has been identified, and in determining whether 2pbl shared similar functionality, conservation of the catalytic triad between the two proteins was assessed (see figure 1).
Figure 1: Conservation of the ... catalytic triad between 2cb7 and 2pbl.
Evidence from Similar Sequences
Genomic Context
Cellular Context (location)
Species Similarities