Chromosome 1 open reading frame 41 Function: Difference between revisions
No edit summary |
No edit summary |
||
| Line 4: | Line 4: | ||
[[Image:GO.png|centre|framed| | [[Image:GO.png|centre|framed|]] | ||
| Line 17: | Line 17: | ||
2. Cytoprotective Function | 2. Cytoprotective Function | ||
sHsp inhibit cell deaths by: | |||
* Stabilizing the mitochondria system | |||
sHsp interact with various component of programmed cell death machinery. It prevent the release of proapoptotic protein (caspase-3 and cytochrome c)within the mitochondria. | |||
* Increasing the Akt phosphorylation. | |||
Akt is a cytoprotective protein kinase which inactivates the tumor suppressor through phosphorylation-dependent process. | |||
Based on the structure analysis, C1orf41 has a loop which binds to Ca2+. | |||
The presence of Ca2+ presumably related to Akt phosphorylation (support the phosphorylation). | |||
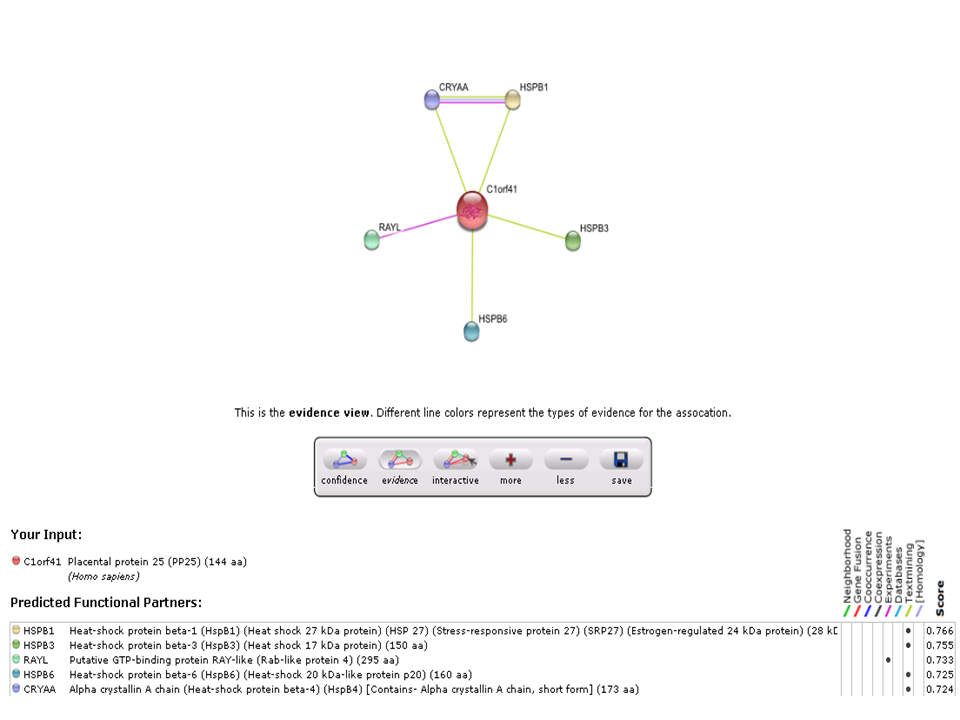
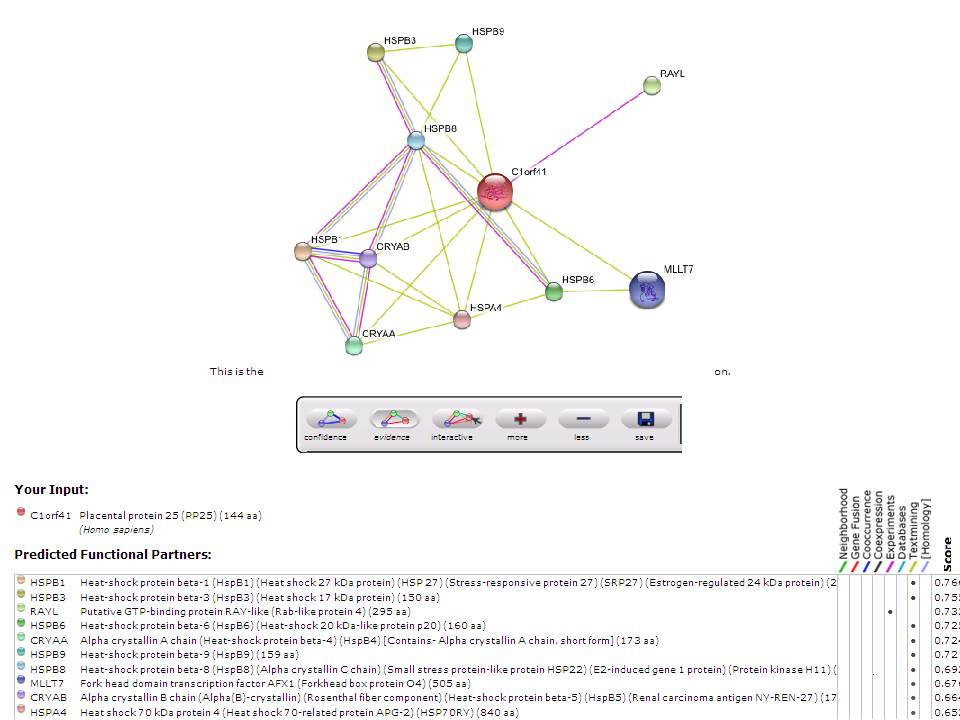
Based on experimental evidence, there was an interaction between c1orf41 and RAYL (Ras-like-GTPase superfamily member). | |||
[[Image:string-evidence view.png|centre|framed| | [[Image:string-evidence view.png|centre|framed|]] | ||
Revision as of 00:20, 16 June 2009
C1orf41
Based on Blast result, our protein has high similarity with sHsp, specifically with sHsp 16.2. Thus, they may have similar function.
sHsp function:
1. Molecular chaperone function
Under cellular stress (heat or chemical stress), sHsp will prevent protein aggregation and precipitation by interact with & stabilize the targeted protein.
Bellyei et al(2007) tested the chaperoning function of sHsp 16.2. They determined the thermal resistance of E. coli over-expressing sHsp 16.2 or aHsp27. The result showed that the sHsp 16.2 over expressing E. coli were resistant against heat stress (increase number of surviving colonies) compared to E. coli not expressing sHsp.
2. Cytoprotective Function
sHsp inhibit cell deaths by:
- Stabilizing the mitochondria system
sHsp interact with various component of programmed cell death machinery. It prevent the release of proapoptotic protein (caspase-3 and cytochrome c)within the mitochondria.
- Increasing the Akt phosphorylation.
Akt is a cytoprotective protein kinase which inactivates the tumor suppressor through phosphorylation-dependent process.
Based on the structure analysis, C1orf41 has a loop which binds to Ca2+. The presence of Ca2+ presumably related to Akt phosphorylation (support the phosphorylation).
Based on experimental evidence, there was an interaction between c1orf41 and RAYL (Ras-like-GTPase superfamily member).
Some articles about HSPB11 suggest that small heat shock protein play a role in development of cancer. Small hsp has anti apoptotic activity which if oer expressed lead to tumor growth and resistance to chemo or radiotheraphy (Bellyei et al, 2006; Pozsgai et al, 2007). The anti apoptotic effect of hsp is mediated by the activation of Hsp90 (which Hsp 16.2 binds with) and by the activation of PI-3 kinase-Akt pathway. Pozsgai et al (2007) research data suggest that there is correlation between the progressive cytolasmic of small hsp and brain tumor malignancy.
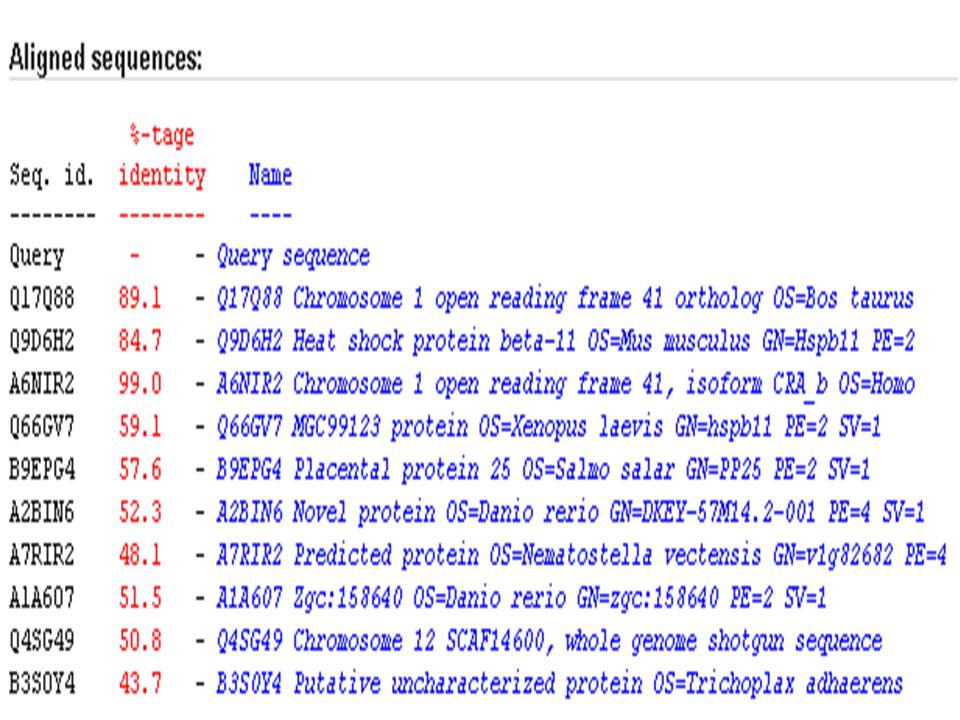
The result of Blast search vs UniProt showed that 1tvg has the highest percentage identity (99 %)to chromosome 1 open reading frame 41, isoform CRA_b of Homo sapiens.
STRING result: Predicted protein-protein interaction
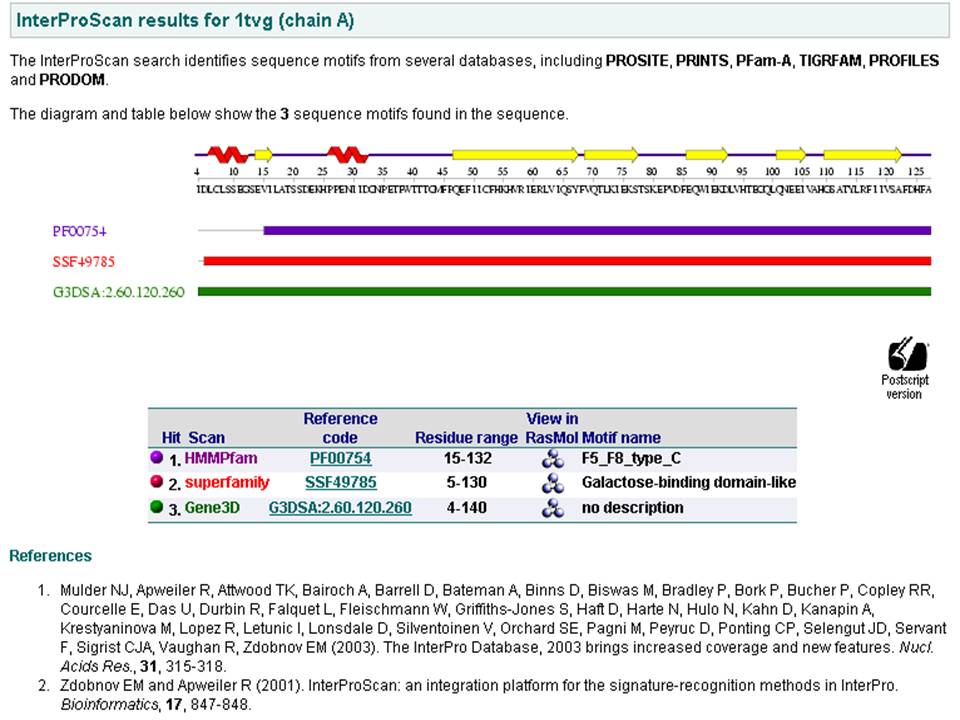
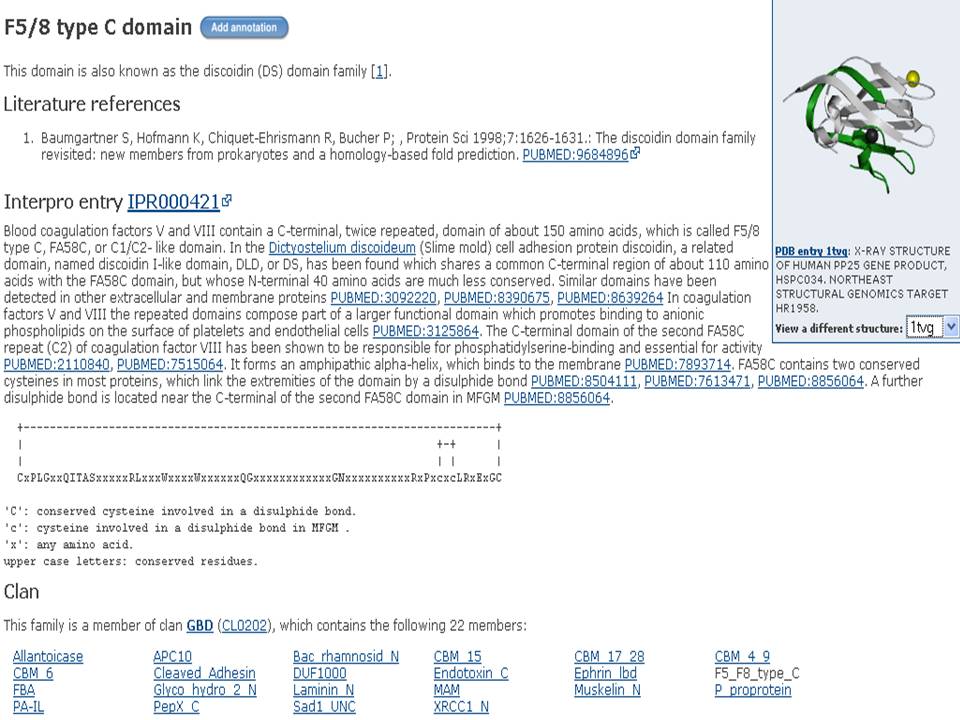
Based on Pfam result, the domain of our protein target (1tvg) is F5/8 (discoidin). Discoidin is blood coagulation factor V and VIII which contain in C terminal.