Chromosome 1 open reading frame 41 Function: Difference between revisions
No edit summary |
No edit summary |
||
| (13 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
'''C1orf41''' | '''C1orf41''' | ||
Based on Blast result, our protein has high similarity with sHsp, specifically with sHsp 16.2. | *Based on Blast result, our protein has high similarity with sHsp, specifically with sHsp 16.2. | ||
*Based on Pfam result, our protein target is a member of F5/8 type C (discoidin)domain. | |||
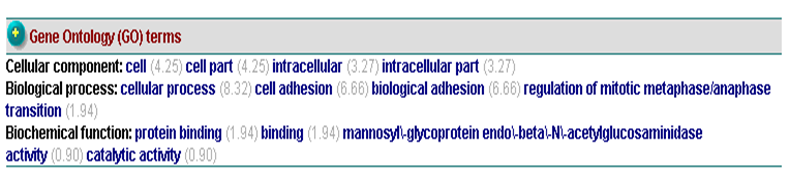
[[Image:GO.png|centre|framed|]] | |||
'''sHsp function:''' | '''sHsp function:''' | ||
1. Molecular chaperone function | 1. Molecular chaperone function | ||
Under cellular stress (heat or chemical stress), sHsp will prevent protein aggregation and precipitation by interact with & stabilize the targeted protein. | Under cellular stress (heat or chemical stress), sHsp will prevent protein aggregation and precipitation by interact with & stabilize the targeted protein. | ||
| Line 12: | Line 17: | ||
2. Cytoprotective Function | 2. Cytoprotective Function | ||
sHsp inhibit cell deaths by: | |||
* Stabilizing the mitochondria system | |||
sHsp interact with various component of programmed cell death machinery. It prevent the release of proapoptotic protein (caspase-3 and cytochrome c)within the mitochondria. | |||
* Increasing the Akt phosphorylation. | |||
Akt is a cytoprotective protein kinase which inactivates the tumor suppressor through phosphorylation-dependent process. | |||
Based on the structure analysis, C1orf41 has a loop which binds to Ca2+. | |||
The presence of Ca2+ presumably related to Akt phosphorylation (support the phosphorylation). | |||
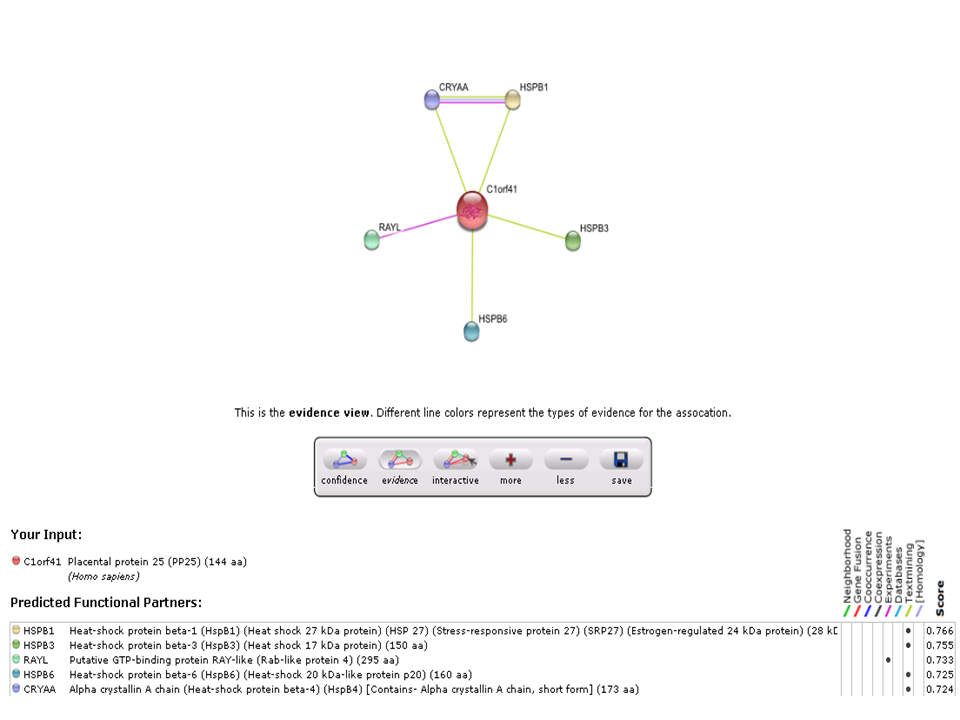
Based on experimental evidence, there was an interaction between c1orf41 and RAYL (Ras-like-GTPase superfamily member). | |||
[[Image:string-evidence view.png|centre|framed|]] | |||
* Overexpression of Ras GTPase activating protein will enhance the phosphorylation & activity of Akt. | |||
'''sHsp play as antiapoptotic protein. Thus, it is highly express in cancer''' | |||
----- | |||
'''Discoidin''' | |||
"Proteins from discoidin domain commonly function as cellsurface-attached carbohydrate residues, proteins and lipids' (Hidai ''et al'', 2007). | |||
*Discoidin first described as lectins with high affinity for galactose | |||
New member: | |||
*Discoidin factor V and VIII, functions as coagulation factor | |||
*F5/8 type C domain, function as cell-cell interaction and recognition | |||
'''However, discoidin is an extracellular protein, while c1orf41 is intracellular. Thus, c1orf41 is more likely a sHsp.''' | |||
[[Chromosome 1 open reading frame 41|Back to main page]] | [[Chromosome 1 open reading frame 41|Back to main page]] | ||
Latest revision as of 05:09, 16 June 2009
C1orf41
- Based on Blast result, our protein has high similarity with sHsp, specifically with sHsp 16.2.
- Based on Pfam result, our protein target is a member of F5/8 type C (discoidin)domain.
sHsp function:
1. Molecular chaperone function
Under cellular stress (heat or chemical stress), sHsp will prevent protein aggregation and precipitation by interact with & stabilize the targeted protein.
Bellyei et al(2007) tested the chaperoning function of sHsp 16.2. They determined the thermal resistance of E. coli over-expressing sHsp 16.2 or aHsp27. The result showed that the sHsp 16.2 over expressing E. coli were resistant against heat stress (increase number of surviving colonies) compared to E. coli not expressing sHsp.
2. Cytoprotective Function
sHsp inhibit cell deaths by:
- Stabilizing the mitochondria system
sHsp interact with various component of programmed cell death machinery. It prevent the release of proapoptotic protein (caspase-3 and cytochrome c)within the mitochondria.
- Increasing the Akt phosphorylation.
Akt is a cytoprotective protein kinase which inactivates the tumor suppressor through phosphorylation-dependent process.
Based on the structure analysis, C1orf41 has a loop which binds to Ca2+. The presence of Ca2+ presumably related to Akt phosphorylation (support the phosphorylation).
Based on experimental evidence, there was an interaction between c1orf41 and RAYL (Ras-like-GTPase superfamily member).
- Overexpression of Ras GTPase activating protein will enhance the phosphorylation & activity of Akt.
sHsp play as antiapoptotic protein. Thus, it is highly express in cancer
Discoidin
"Proteins from discoidin domain commonly function as cellsurface-attached carbohydrate residues, proteins and lipids' (Hidai et al, 2007).
- Discoidin first described as lectins with high affinity for galactose
New member:
- Discoidin factor V and VIII, functions as coagulation factor
- F5/8 type C domain, function as cell-cell interaction and recognition
However, discoidin is an extracellular protein, while c1orf41 is intracellular. Thus, c1orf41 is more likely a sHsp.