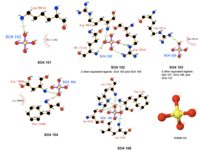
Discussion of SNAPG

The SNAPG structure obtained Danio rerio possess 4 chains of alpha helical hairpins fold without any beta strands observed. Each chain was interconnected with one another via hydrogen bond and non-bonded contacts (PDBSum). The helices towards N-terminal end were connected by extended loops and those towards C-terminal end tend to form a globular bundle presumably forming a cleft. Two different types of ligand interact with SNAPG differently. One of the ligand types, sulfanate ion (SO4), was found to from a distinct interaction with different residues in different chain (Figure 1 & 2). Meanwhile, selenomethione (MSE) ligands were integrated into protein structure as seem to appear as modified amino acid residues (Figure 1).
Although the protein structure of SNAPG was already known, the function is still yet under investigations. Protein super families identification reveals that SNAPG is made of 2 domains in which both of the domains had a soluble-NFS-attachment-protein (SNAP) activity. The sequence conservation against the protein super families suggests that SNAP proteins have a conserved 3D shape (need ref). Structural alignment against a representative structure in PDB databases using Dali web server shows several proteins with acknowledged protein function and domains shares structure similarity with SNAPG protein (Table 1). For this study purpose, two proteins with highest Z-value were chosen. A vesicular transport protein sec17 found in yeast and type 4 fimbrial biogenesis protein pili in Pseudomonas aeruginosa had a Z-value of 23.3 and 12.9; and shares 23 and 14 respectively %identity with SNAPG. Structure-based alignment of these structures indicates similar secondary structure and motifs but the amino acid conservation were not satisfying (Figure 4&5) suggesting the function can not be determined based on structure alignment alone.
nanti aku mau jelasin ttg ligandnya
Tea: evolution linked with function.
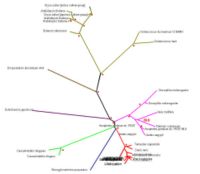
SNAP-gamma protein evolutionary tree mostly contains more than 75% boostrapvalue. Thus, red dot signs was assign to represent more than 75% boostrap value. The value was indicated the degree of confidence about the branching order. As an example: in the diagram phylogenetic tree (see Figure 3), there is 39.0 boostrap value in branching Apis melifera and Tribolium castaneum. The boostrap value is below 75%, thus it could be concluded that the degree of confidence of branching tree in the correct order is far below 95%. On the other hand, boostrap value more than 75% or 75.00 means 95% confidence that the branching tree is in the correct order
As indicated in diagram in Figure 3, SNAP-gamma protein was evolved in eukaryotes. There is no prokaryotes organism detected in the phylogenetic tree.
Function (in various organism) = nunggu justine
Thus, it could be concluded that SNAP-gamma protein is an important protein in eukaryotes animal.