Evolution: Difference between revisions
JasonCheong (talk | contribs) |
JasonCheong (talk | contribs) No edit summary |
||
| Line 85: | Line 85: | ||
From these short conservation regions, the functions or even structure of the encoded proteins could have significance in its evolutionary pattern. | From these short conservation regions, the functions or even structure of the encoded proteins could have significance in its evolutionary pattern. | ||
=='''Phylogenetic Map (Tree)'''== | =='''Phylogenetic Map (Tree)'''== | ||
| Line 107: | Line 109: | ||
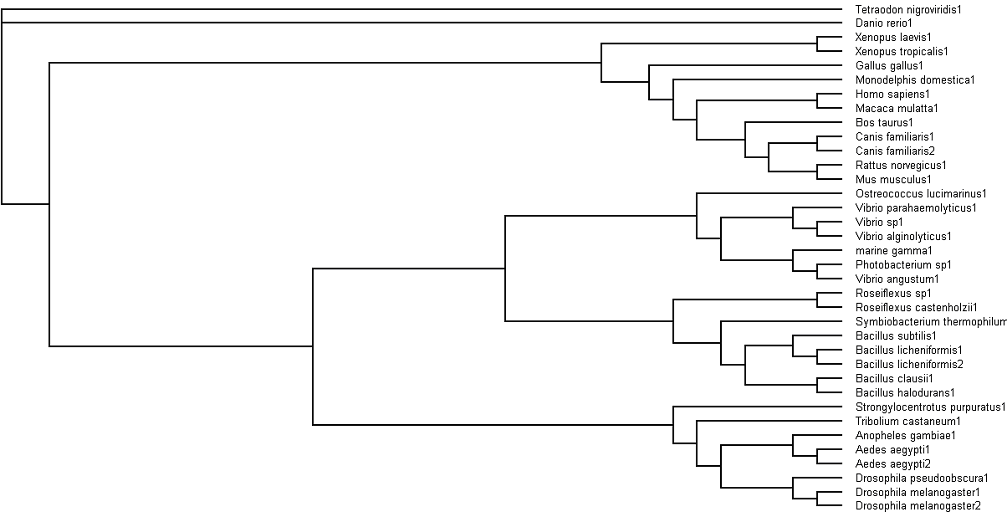
'''Fig. 3: Phylogenetic tree''' showing organisms with related protein sequence homology in Radial Tree view (top); and Rectangular Cladogram view (bottom) | '''Fig. 3: Phylogenetic tree''' showing organisms with related protein sequence homology in Radial Tree view (top); and Rectangular Cladogram view (bottom) | ||
From the Rectangular Cladogram view, it could be observed that there are '''four distinct separate groups'''. | From the Rectangular Cladogram view, it could be observed that there are '''four distinct separate groups'''. | ||
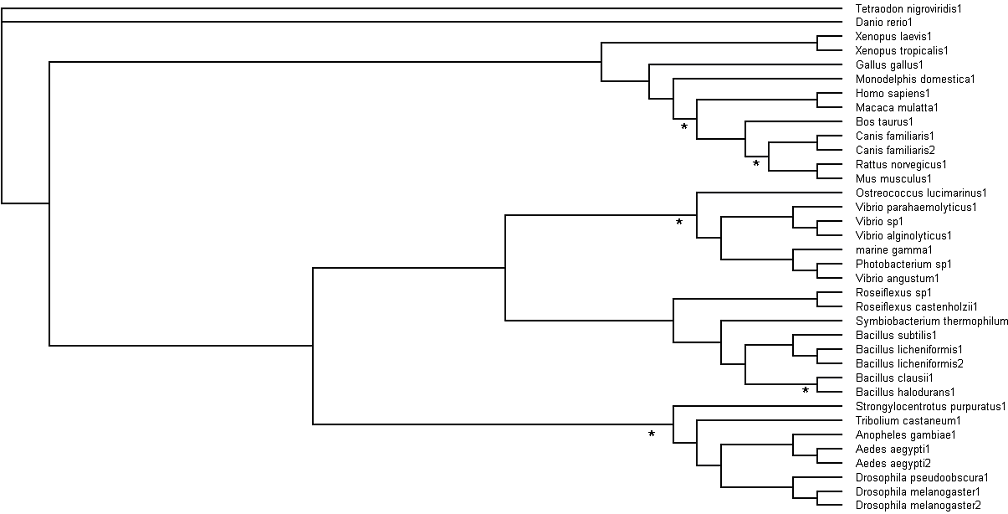
=='''Bootstrapping'''== | |||
See '''[[Materials and Methods]]''' | |||
[[Image:bootstrap1.png|Bootstrap Values]] | |||
Revision as of 19:22, 10 June 2007
Sequence Homology
The following query protein sequence of N-acetylneuraminic Acid was obtained from GenBank:
| mgsdkihhhh hhmglsrvra vffdldntli dtagasrrgm levikllqsk yhykeeaeii
cdkvqvklsk ecfhpystci tdvrtshwee aiqetkggad nrklaeecyf lwkstrlqhm iladdvkaml telrkevrll lltngdrqtq rekieacacq syfdaivigg eqkeekpaps ifyhccdllg vqpgdcvmvg dtletdiqgg lnaglkatvw inksgrvplt sspmphymvs svlelpallq sidckvsmsv |
Fig. 1: Amino Acid Sequence (260 aa) of N-acetylneuraminic Acid (2gfh) protein from Mus musculus (House Mouse)
A total of 500 proteins were yielded.
Only a 38 proteins were selected for comparison as these had shown higher homology to the query sequence, in contrast with the remainder of the search results.
These proteins were chosen according to their bit scores and E-values. Two more outlier partial sequences contributing to poor overall alignment (huge deletion gaps) were subsequently removed. The remaining 36 sequences were used for the generation of the phylogenetic tree (and bootstrapped tree as well).
The bacteria sequence matches used for multiple sequence analysis and phylogenetic tree mapping were representative of the other baterial sequences not selected. These selected sequences had the highest bit scores and E-values.
Multiple Sequence Alignments (msa)
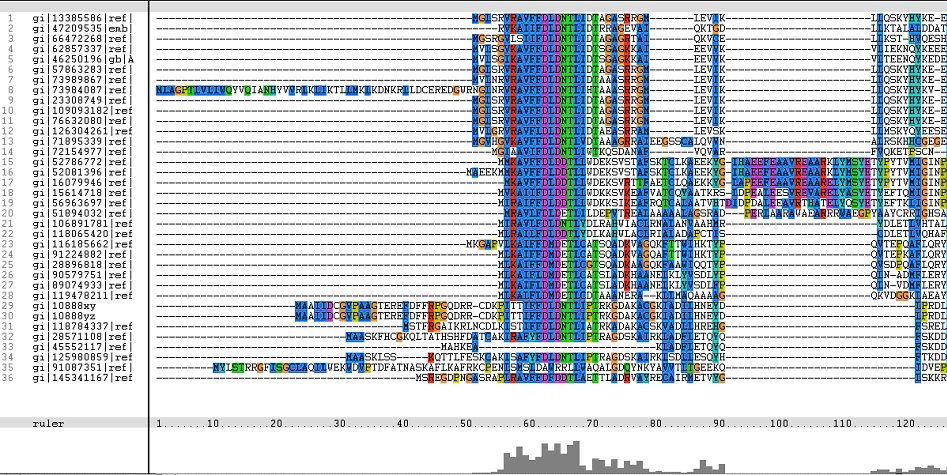
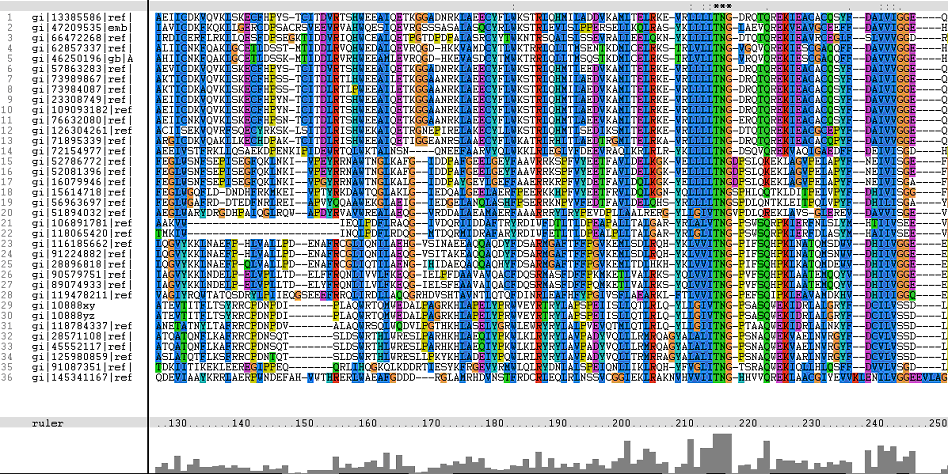
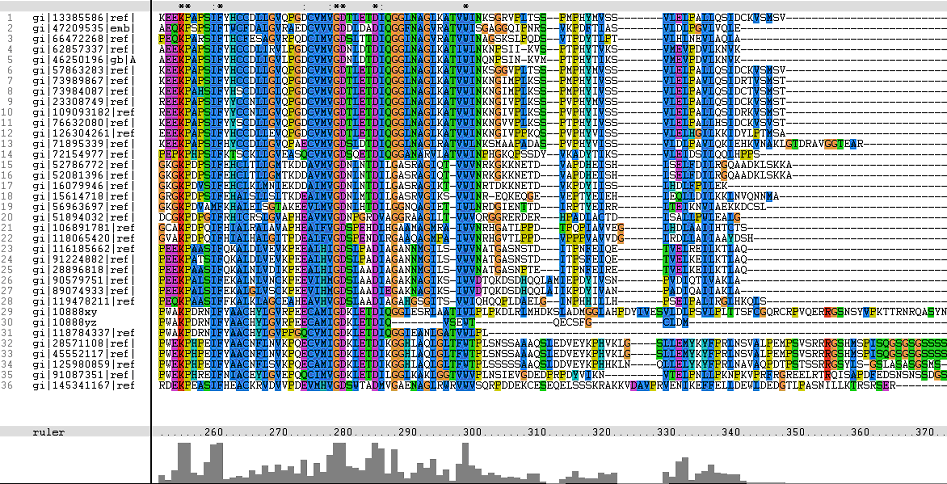
The following sequence alignment was obtained (Fig. 2)
From the alignments, gi|10888xy and gi|10888yz are representative of gi|108881764 and gi|108881765 respectively. Both these hypothetical proteins belong to the mosquito Aedes aegypti.
The identifier numbers for these two proteins were initially changed to an alpha-numeric one, due to the inability of Phylip to generate a tree from the original identifiers. This was due to the fact that the programme only took the first five numeric digits (10888), thereby resulting in a programme error prompt which listed both proteins as duplicates (from the identifier numbers). Both these identifiers were subsequently renamed for the final phylogenetic tree.
The topmost sequence belongs to the query protein.
Figure 1. Multiple Sequence Alignments of 2gfh amino acid sequences with that of other similar protein sequences in various organisms.
From the multiple sequence alignment, it can be observed that there are domain conservations throughout the protein sequences. Small insertion and deletion gaps were noticeable along the alignment as well. A particularly large insertion gap was observed between amino acids 91 to 114.
The organisms with the large insertion gaps were as identified below:
- Bacillus licheniformis
- Bacillus subtilis
- Bacillus halodurans
- Bacillus clausii
- Symbiobacterium thermophilum
A highly conserved (with invariant) section of amino acids (LV)–(LVA)–(LIV)–(LIV)-T-N-G was observed in all the sequences from amino acid 211 to 217 in the alignment. Downstream of this conserved portion of genes are 5 more invariant positions (1 or 2 amino acids in length).
From these short conservation regions, the functions or even structure of the encoded proteins could have significance in its evolutionary pattern.
Phylogenetic Map (Tree)
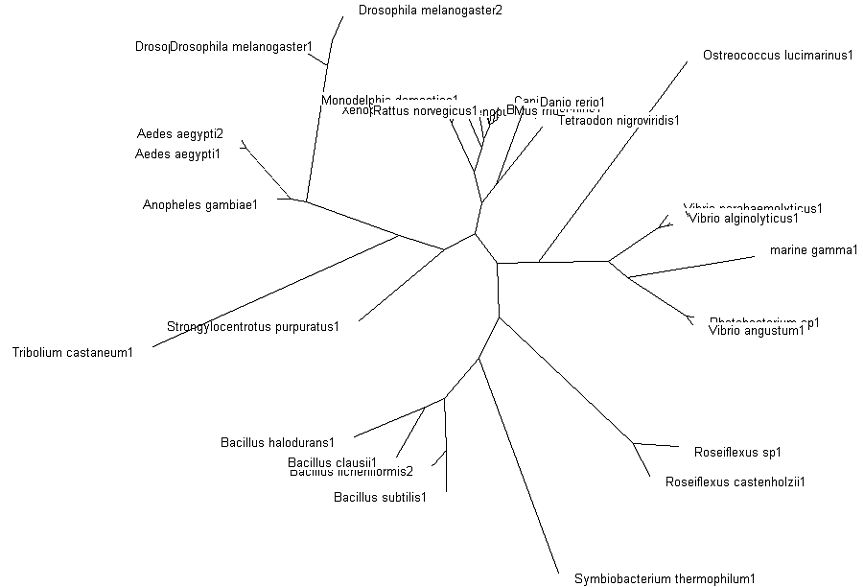
The tree was plotted to obtain the phylogenetic lineage (Fig. 3):
Fig. 3: Phylogenetic tree showing organisms with related protein sequence homology in Radial Tree view (top); and Rectangular Cladogram view (bottom)
From the Rectangular Cladogram view, it could be observed that there are four distinct separate groups.
Bootstrapping
| Catalytic activity | N-acylneuraminate 9-phosphate + H2O = N-acylneuraminate + phosphate |
| Cofactor | Magnesium (By similarity) |
| Enzyme regulation | Inhibited by vanadate and calcium (By similarity) |
| Pathway | Carbohydrate metabolism; aminosugar metabolism |
| Similarity | Belongs to the haloacid dehalogenase-like hydrolase superfamily. NANP family |