Fascin Function: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
The fascins are a structurally unique and evolutionarily conserved group of actin cross-linking proteins. They are a 54-58Kda protein that was originally isolated from sea urchin egg (Strongylocentrotus purpuratus) but is also found in drosophila and vertebrates including man. Fascins function in the organisation of two major forms of actin-based structures: dynamic, cortical cell protrusions and cytoplasmic microfilament bundles. Cell protrusions are extensions of the plasma membrane that are supported internally by actin-based structures that impart mechanical stiffness. The cortical structures, which include filopodia, spikes, lamellipodial ribs, oocyte microvilli and the dendrites of dendritic cells, have roles in cell-matrix adhesion, cell interactions and cell migration, whereas the cytoplasmic actin bundles appear to participate in cell architecture and intracellular movements [1]. The fascin–actin interaction is under complex regulation from the extracellular matrix, peptide factors and other actin-binding proteins. | The fascins are a structurally unique and evolutionarily conserved group of actin cross-linking proteins. They are a 54-58Kda protein that was originally isolated from sea urchin egg (Strongylocentrotus purpuratus) but is also found in drosophila and vertebrates including man. Fascins function in the organisation of two major forms of actin-based structures: dynamic, cortical cell protrusions and cytoplasmic microfilament bundles. Cell protrusions are extensions of the plasma membrane that are supported internally by actin-based structures that impart mechanical stiffness. The cortical structures, which include filopodia, spikes, lamellipodial ribs, oocyte microvilli and the dendrites of dendritic cells, have roles in cell-matrix adhesion, cell interactions and cell migration, whereas the cytoplasmic actin bundles appear to participate in cell architecture and intracellular movements [1]. The fascin–actin interaction is under complex regulation from the extracellular matrix, peptide factors and other actin-binding proteins. | ||
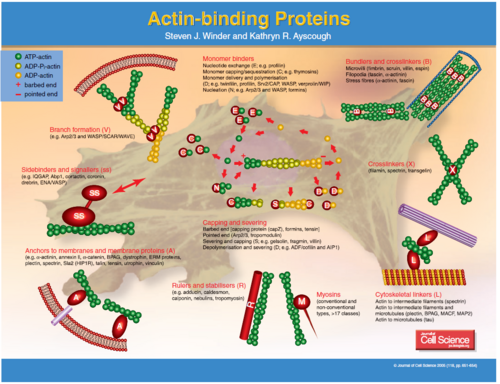
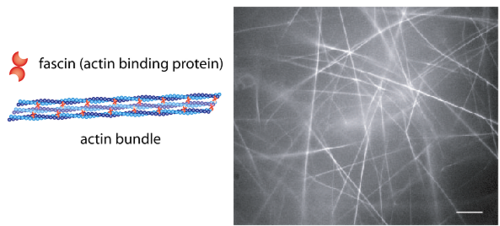
[[image:Untitled4.PNG|left|thumb|500px|'''Figure 1''': Image showing various actin-bundling proteins (ABP) working on a whole in a cell.]][[image:Untitled.PNG|right|thumb|500px|'''Figure 1''' Figure 1. Fig. 1: Schematic view of an actin bundle (left). ABPs such as fascin organize actin filaments in a parallel fashion. In vitro these actin/fascin bundles create a purely bundled network (right, scale bar is 10 µm). ]] | [[image:Untitled4.PNG|left|thumb|500px|'''Figure 1''': Image showing various actin-bundling proteins (ABP) working on a whole in a cell.]][[image:Untitled.PNG|right|thumb|500px|'''Figure 1''' Figure 1. Fig. 1: Schematic view of an actin bundle (left). ABPs such as fascin organize actin filaments in a parallel fashion. In vitro these actin/fascin bundles create a purely bundled network (right, scale bar is 10 µm). ]]<br><br><br><br><br><br> | ||
Revision as of 11:08, 11 June 2009
The fascins are a structurally unique and evolutionarily conserved group of actin cross-linking proteins. They are a 54-58Kda protein that was originally isolated from sea urchin egg (Strongylocentrotus purpuratus) but is also found in drosophila and vertebrates including man. Fascins function in the organisation of two major forms of actin-based structures: dynamic, cortical cell protrusions and cytoplasmic microfilament bundles. Cell protrusions are extensions of the plasma membrane that are supported internally by actin-based structures that impart mechanical stiffness. The cortical structures, which include filopodia, spikes, lamellipodial ribs, oocyte microvilli and the dendrites of dendritic cells, have roles in cell-matrix adhesion, cell interactions and cell migration, whereas the cytoplasmic actin bundles appear to participate in cell architecture and intracellular movements [1]. The fascin–actin interaction is under complex regulation from the extracellular matrix, peptide factors and other actin-binding proteins.
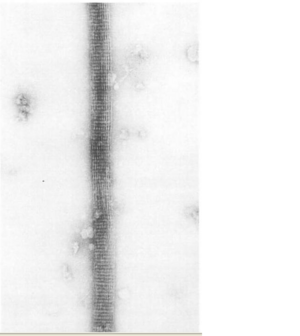
The ultrastructure of actin-fascin needles was described by Kane (1975, 1976) using optical diffraction and image-reconstruction methods. Each needle consisted of a bundle of fascicle with approximate parallel actin filaments arranged in a hexagonal arrangement with 8.3nm spacing. It was found from this that the links in the actin-fascin needle are not evenly spaced along the monomer but occur alternatingly at every 4 and 5 actins (Bryan & Kane, 1978).