|
|
| (40 intermediate revisions by 3 users not shown) |
| Line 1: |
Line 1: |
| __TOC__
| | The predicted function based on the evolution and structure |
|
| |
|
| =='''Proposed Functions'''== | | <font size = "4">'''Hydrolase'''</font> |
|
| |
|
| * Hydrolase Activity
| |
|
| |
|
| * Magnesium Ion Binding
| | [[Image:Document18_01.png]] |
|
| |
|
| * N-acylneuraminate-9-phosphatase Activity
| | Hydrolyase catalyze the hydrosis of the chemical bond between A and B, resulting of 2 simple molecules |
|
| |
|
| * Phosphoglycolate Phosphatase Activity
| |
|
| |
|
| | * Hydrolase |
| | ** Catalyze hydrolysis reaction |
| | ** Addition of the hydrogen and hydroxyl ions of water |
| | ** Splitting into 2 or more simpler molecules |
| | ** EC class 3 |
|
| |
|
| [http://amigo.geneontology.org/cgi-bin/amigo/go.cgi?view=details&depth=1&query=0016787 1. Hydrolase Activity]
| |
|
| |
|
| [http://amigo.geneontology.org/cgi-bin/amigo/go.cgi?view=details&depth=1&query=0000287 2. Magnesium Ion Binding]
| |
|
| |
|
| [http://amigo.geneontology.org/cgi-bin/amigo/go.cgi?view=details&depth=1&query=0050124 3. N-acylneuraminate-9-phosphatase Activity]
| | == Function of sulfatases == |
|
| |
|
| [http://amigo.geneontology.org/cgi-bin/amigo/go.cgi?view=details&depth=1&query=0008967 4. Phosphoglycolate Phosphatase Activity]
| | Sulfatases are enzymes,which hydrolyse sulfate ester bonds of substrates. |
| | Most of the family members has shown to contain a highly conserved cystine residue and a bivalent metal binding site. |
|
| |
|
|
| |
|
|
| |
|
| {| border="1" cellpadding="15" cellspacing="0"
| |
| |+'''Table of functions'''
| |
| |Catalytic activity
| |
| |N-acylneuraminate 9-phosphate + H2O = N-acylneuraminate + phosphate
| |
| |-
| |
| |Cofactor
| |
| |Magnesium (By similarity)
| |
| |-
| |
| |Enzyme regulation
| |
| |Inhibited by vanadate and calcium (By similarity)
| |
| |-
| |
| |Pathway
| |
| |Carbohydrate metabolism; aminosugar metabolism
| |
| |-
| |
| |Similarity
| |
| |Belongs to the haloacid dehalogenase-like hydrolase superfamily. NANP family
| |
| |}
| |
|
| |
|
|
| |
|
| | == Functional site== |
| | MSA data revealed some conserved residues on the sequence. They were mapped on the three dimantional structure. |
|
| |
|
| | [[Image:Zn_Cl_surface]] |
|
| |
|
| =='''GO Terms'''==
| |
|
| |
| Polymer: haloacid dehalogenase-like hydrolase domain containing 4
| |
|
| |
| Molecular Function: None
| |
|
| |
| Biological Process: None
| |
|
| |
| Cellular Component: None
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| ----
| |
|
| |
| info
| |
|
| |
| N-acetylneuraminic acid phosphatase
| |
|
| |
| Homologous to mouse (Mus musculus)
| |
|
| |
| Haloacid Dehalogenase Like Hydrolase Domain Containing 4
| |
|
| |
| Classified as Hydrolase
| |
|
| |
| [http://www.ebi.ac.uk/thornton-srv/databases/profunc/index.html Infomation on ProFunc] (useful 2gfhA)
| |
|
| |
| [http://www.ebi.ac.uk/pdbsum/ Information on PDBsum] (2gfh)
| |
|
| |
| From [http://www.ebi.ac.uk/cgi-bin/sumtab?tool=fasta&jobid=fasta-20070515-07270763 FASTA]
| |
| most likely function is N-acetylneuraminic acid phosphatase.
| |
|
| |
| The haloalkanoate dehalogenase superfamily (HADSF) is one of the largest and most ubiquitous enzyme families identified to date, with over 3,000 members in organisms ranging from bacteria to humans. Remarkable diversity of chemistry and function has emerged through evolution of the HAD catalytic scaffold. Despite the name, the dehalogenases, which catalyze carbon group transfer, represent a minute fraction of the family. All other known catalytic activities are directed at phosphoryl transfer. Numerous proteins from the HADSF are found in each organism (29 in E. coli and 58 in humans, for example) where they perform a diverse collection of novel physiological functions in primary and secondary metabolism, membrane transport, signal transduction, and nucleic acid repair. http://biophysics.bumc.bu.edu/faculty/allen/allenpage/had.htm
| |
| ---- | | ---- |
|
| |
|
| Press here to go [http://compbio.chemistry.uq.edu.au/mediawiki/index.php/BIOL3004_2007 ''Back'']
| | Click here to go [http://compbio.chemistry.uq.edu.au/mediawiki/index.php/BIOL3004_2007 ''Back''] |
The predicted function based on the evolution and structure
Hydrolase
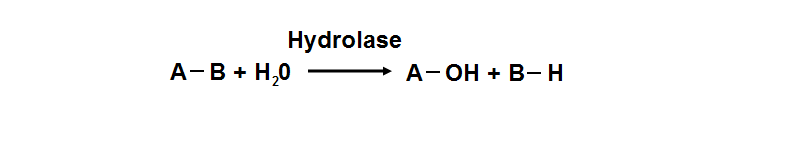
Hydrolyase catalyze the hydrosis of the chemical bond between A and B, resulting of 2 simple molecules
- Hydrolase
- Catalyze hydrolysis reaction
- Addition of the hydrogen and hydroxyl ions of water
- Splitting into 2 or more simpler molecules
- EC class 3
Function of sulfatases
Sulfatases are enzymes,which hydrolyse sulfate ester bonds of substrates.
Most of the family members has shown to contain a highly conserved cystine residue and a bivalent metal binding site.
Functional site
MSA data revealed some conserved residues on the sequence. They were mapped on the three dimantional structure.
File:Zn Cl surface
Click here to go Back
