Hypothetical protein Discussion: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
[[Image:conres.jpg]] | [[Image:conres.jpg]] | ||
The hypothetical puitive sugar binding protein 2ob5 is part of the RbsD/FucU superfamily in particular it appears to be a FucU protein. This was found through combination of evolutionary and structural analysis compared to existing literature. | The hypothetical puitive sugar binding protein 2ob5 is part of the RbsD/FucU superfamily in particular it appears to be a FucU protein. This was found through combination of evolutionary and structural analysis compared to existing literature. | ||
Revision as of 22:33, 15 June 2009
The hypothetical puitive sugar binding protein 2ob5 is part of the RbsD/FucU superfamily in particular it appears to be a FucU protein. This was found through combination of evolutionary and structural analysis compared to existing literature.
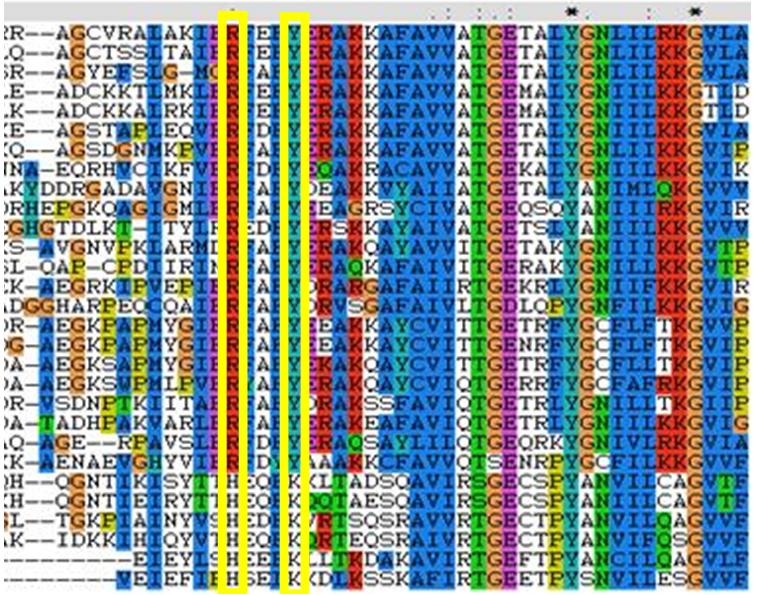
Structural alignments found that the target protein matched well against ribose, however there was no fucose to compare it to. This is a limit to the structural comparisons that can be made. From the structural alignments we assume it forms a decamerichal olligoma and may contain putative chloride binding motif involved with polymerisation. These theories are based on the assumption that there is no significant difference between tertiary structures between FucU and RbsD. This is a valid assumption because the secondary structures are so similar. The 1ogd binding pocket consists of, D-28, Y-120, N-122, H-98 and K-102. It also contains H-20 from a neighboring monomer. The 2ob5A binding pocket is similar it has the H-22 from the other protein and D-30, Y-136, There is no corresponding N-122. The H-98 and The K-102 were substituted with R-114 and Y-118 respectively. The R and Y substitution has been found according to literature to be conserved in fucose homologues. This corresponds to our MSA, see figure 1. This also shows that our protein is a fucose binding protein.
The crystallographic structure of 1ogD contains a metal-ion coordinating pocket formed by the interface between subunits adjacent between upper and lower sections of the ring. This ion – potentially a Cl- - is cocrystallised in the structure of 1ogD, and appears to be coordinated by the Lys2 residue. This metal ion would have a potential stabilising effect on the oligomer. Visual characteristics of the corresponding 2ob5 region suggest that 2ob5 also exists as a decamer. However, the chloride-coordinating Lysine is replaced by an aliphatic leucine in 2ob5 and all other fucose-binding proteins analysed (complete alignment). From this, we hypothesise that the chloride ion is absent in fucose-binding proteins, but that the proteins are still able to assume a stable decameric ring oligomer.
Our query protein was found in several alpha-proteobacteria (high similarity), supported by a bootstrap value of 100 (tree, blue). The chordate group was monophyletic (red, bootstrap value of 99), but sister group to several bacteria (yellow, bootstrap value of 75). We could not find any significant differences distinguishing these bacteria from the bacteria that were not sister group to chordates (purple).This raises a question about the evolution of our query protein: why do some bacteria have transporters that are more similar to eukaryotic proteins than to other bacteria? In addition, we found a significantly divergent group of sequences that was completely separate from the remainder of sequences (green, bootstrap 100). This was attributed to the type of sugar targeted by the protein transporter. It may be hypothesized that the gene encoding the RbsD transporter had undergone duplication in a common ancestor, as indicated by the presence of both these proteins (which are highly divergent) in the bacterium E. coli. Furthermore, we did not encounter any RbsD transporter in eukaryotes.
The RbsD acts as a mutarotase to convert beta purine to beta furine. A proposed mode of action for this role of RbsD mutarotase can be seen in the figue(?). The His20 residue protonates the O5 and the His106 deprotonates the OH attached to the C1. This causes ring-breakage allowing the formation of an equilibrium between the furan and pyran forms. The FucU is fundamentally different, changing the beta to alpha pyranse. The altered residues in the binding site might play a critical role in anomeric conversion of L-fucose by associating with the OH group at C-1 position of the sugar.
Previous research has shown that although RbsD did not show any activity for L-fucose as a substrate FucU exhibited a pyranase activity for D-ribose. This could be a result of evolutionary changes within the protein. RbsD is an ancestor of FucU, this is supported by FucU retaining the ability to interact with limited amounts of D-ribose.
Abstract | Introduction | Method |
Results | Discussion | Conclusion | References