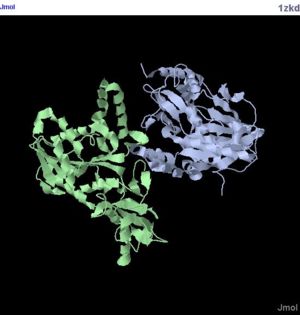
Introduction (1zkd)
Rhodopseudomonas palustris
Rhodopseudomonas palustris is a tropical bacterium from the alpha proteobacteria family. It’s a purple photosynthetic bacterium which is commonly found in the soils and water. It is highly versatile metabolically. As it can survive using any of the four modes of metabolism that support life
• Photoautotrophic or photosynthetic – whereby it gains energy from light and carbon from carbon dioxide
• Photoheterotrophic – whereby it gains energy from light and carbon from organic compounds
• Chemoheterotrophic – whereby it gains carbon and energy from organic compounds
• Chemoautotrophic – whereby it gains energy from inorganic compounds and carbon from carbon dioxide
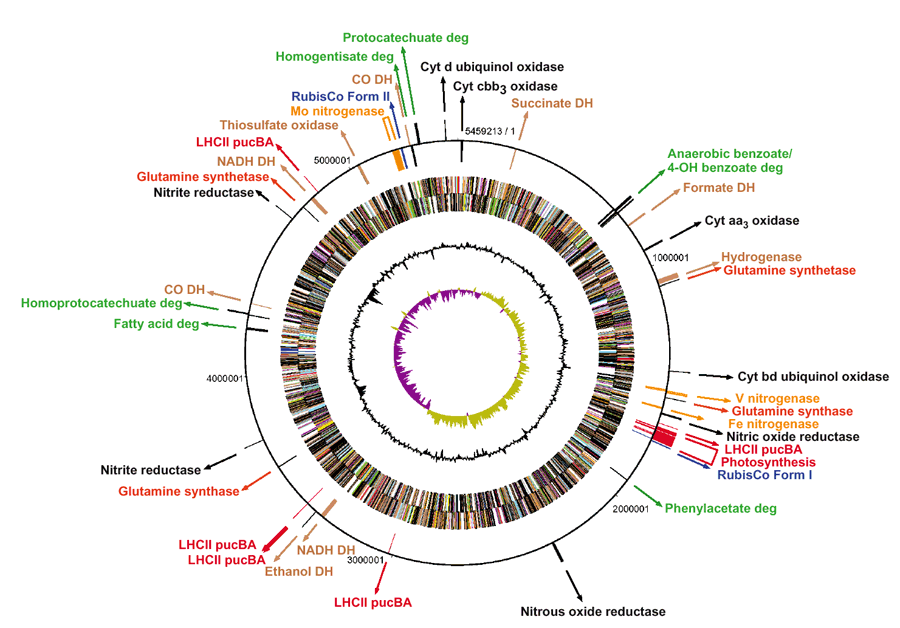
Rhodopseudomonas palustris has a circular chromosome about 5.46Mb long. R.palustris has about 4,836 predicted protein-encoding genes and the bulk of them are used for energy metabolism.
The metabolic group of purple phototrophic bacteria to which it belongs to has been determined as a source of ‘bioplastic’ and for the production of hydrogen which is the by-product of nitrogen fixation.
The image below illustrates how metabolically versatile Rhodopseudomonas palustris bacterial genome is and furthermore what it is known to express thus far.
Protein Identification
Protein can be categorised into its structure, function and evolution. Knowing the structure of the protein allows researchers to predict its function and also helps in fighting diseases that involves proteins. The functions of the protein is important as proteins direct almost all biological functions and by knowing which proteins are responsible for the particular biological functions, drugs can be designed to enhance or inhibit the effect it produces. The drug can use the structural informations of the protein, for example design molecules to fit into the binding site or use the protein structure as a template for drug designs. Function will contain the evolution of the sequence of the expressed proteins as functional residues are more conserved but function may differ in different organism.
There are many methods used to predict structure and function of the protein but evolutionary methods rely on many related sequences, this is also another reason why proteins needs to be identified. Unknown proteins can be used to be compared with known proteins for its structure and functions, therefore less time and resource will be used in identifying proteins.
The 3-D configuration of a protein is important in determining its function. Certain regions of the protein mainly serve to hold certain active regions in the right position, so that they can interact correctly with active regions of other proteins. Methods used in determining the structures include:
1. X-ray Crystallography
2. NMR Spectroscopy
3. Comparative Modelling
Once the structure of the unknown protein is obtained, it can be used to compare with other available structures with known functions as structure is better conserved than sequences. Active sites or binding sites are obtained from the structure and could be used to predict function.
Function prediction is more accurate with the accumulation of evidence from:
1. Sequence by comparing with known proteins
2. Structure - Functions is determined by the structure. The functions might change after duplication or have multiple functions. Therefore combining structural and sequence knowledge help to determine the functions.
3. Genomics Context - Functional units are conserved in bacterial proteins and functionally related proteins get often joint during evolution.
4. Cellular Context - Cellular and sub-cellular as localisation limits the possible function.
5. Evolutionary Context
Our Protein
Using publicly available experimental information, sequence, structure and evolution, the biological function of unknown protein structures can be by analysed. The protein given is named as hypothetical protein LOC55471 isoform 1. The structure of the protein was obtained by using X-ray Diffraction method. The protein comes from the the bacteria Rhodopseudomonas palustris. In this report, we will discuss various methods used and the results obtained. Lastly, the functions of this protein will be predicted.
Sequences
From Mouse
1. mtalvrrcva raglpciwrg kcyssgnepa esnqvtpmlr hlmykikstg pitvaeymke vltnpakgyy vhqdmlgekg dfitspdisq ifgellgvwf vsewiasgks pafqlvelgp grgtltadil rvfsqlgsvl ktcaisihlv evsqklseiq altlaeekvp lerdaeslvy mkgvtksgip vswyrdlkdv pegyslylah effdvlpvhk fqktprgwre vfvdvdpqas dklrfvlapc atpaeafiqr derrehvevc pdagviiqel sqriastgga aliadyghdg tktdtlrgfy ghqlhdvlia pgtadltadv dfsylrrmaq gkvaslgpve qrtflknmgi dvrlkvlldk agepsakqql lggydmlmnp qkmgerfhff allphqrlhg gsqernacqs ktpsssvagf delvwq
From Human
2. msvllrsglg plcavaraai pfiwrgkyfs sgnepaenpv tpmlrhlmyk ikstgpitva eymkevltnp akgyyvyrdm lgekgdfits peisqifgel lgiwfisewm atgkstafql velgpgrgtl vgdilrvftq lgsvlkncdi svhlvevsqk lseiqaltlt kekvplerna gspvymkgvt ksgipiswyr dlhdvpkgys fylaheffdv lpvhkfqktp qgwrevfvdi dpqvsdklrf vlapsatpae afiqhdetrd hvevcpdagv iieelsqria ltggaalvad yghdgtktdt frgfcdhklh dvliapgtad ltadvdfsyl rrmaqgkvas lgpikqhtfl knmgidvrlk vlldksneps vrqqllqgyd mlmnpkkmge rfnffallph qrlqggryqr narqskpfas vvagfselaw q
From Bacteria (Rhodopseudomonas palustris)
3. midqtalate ikrlikaagp xpvwryxelc lghpehgyyv trdplgregd fttspeisqx fgellglwsa svwkaadepq tlrlieigpg rgtxxadalr alrvlpilyq slsvhlvein pvlrqkqqtl lagirnihwh dsfedvpegp avilaneyfd vlpihqaikr etgwhervie igasgelvfg vaadpipgfe allpplarls ppgavfewrp dteilkiasr vrdqggaali idyghlrsdv gdtfqaiash syadplqhpg radltahvdf dalgraaesi garahgpvtq gaflkrlgie tralslxaka tpqvsediag alqrltgegr gaxgsxfkvi gvsdpkietl valsddtdre aerrqgthgl ehhhhhh
Return to Report on 1zkd