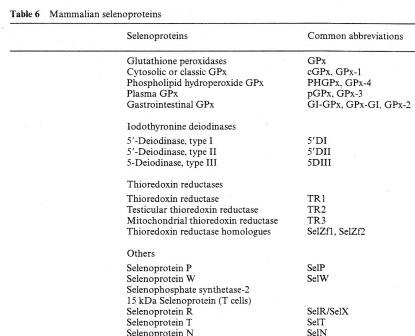
Introduction 2ece
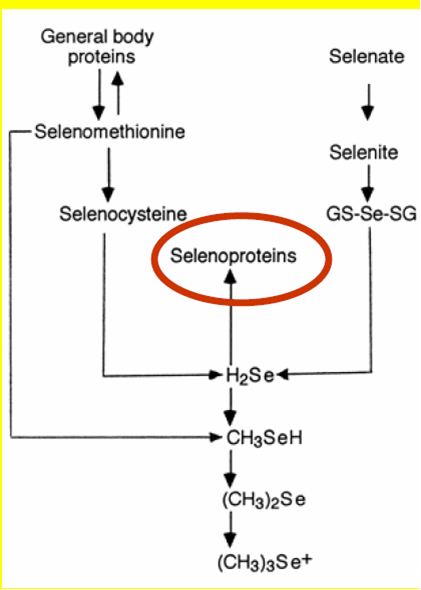
The trace element Selenium (Se) has been known to cause major health problems in livestock and man in soils which are rich in this mineral [Birringer, M., Pilawa, S. and Flohe, L. (2002]). Due to this association of selenium rich soils with these health problems, the beneficial role of this element was severely overlooked until the 1950's when it was discovered to be involved in the synthesis of most enzymes, like redox enzymes Figure 1.1. The roles of selenium into important biochemical reactions was pioneered by Thressa Stadtman selenium biochemistry by the early 1970’s after an observation that bacterial cells required selenium, in their synthesis of an important enzyme, glycine reductase( History of selenium biochemistrySelenium biochemistry video movie file).Further research was carried out and it was found that this element can substitute sulphur in the cysteine amino acid and form a 21st amino acid dubbed as selenocysteine and coded UGA in amino acid codons. Selenium can as well be found in selenomethionine amino acid. Selenium exists in the form of selenate in soil and after absorption it undergoes a series of biochemical steps that involves a passage or binding of one protein to anotherFigure 1.2. It is as well known that selenium in mammals has nutritional benefits and can be transported via haemoglobin and the mechanism and/or protein that mediate its transition from the blood to the whole areas in mammalian body where Selenium plays some important roles has not yet been clearly figured out (Haratake et al, 2008[[1]]) .
Research has long since shifted from the toxicity of selenium and focused on its benefits as it was observed that it is an essential trace element required in human diet and it can be incorporated into amino acids.This advances has led to the discovery of a certain selenium binding protein that does not utilise selenocysteine, but Se behaves as its ligand. People with selenium deficiency were observed to be prone to cancer for exampple selenium deficiency is implicated in the induction of hepatocellular carcinorma due to hepatitis B virus (Birringer, M., et al, 2002), probably due to the fact that selenium is a cofactor in enzymes that are involved in hydroperoxide detoxification and other anti-carcinogenic activities. However, further mutational studies have revealed that this issue is more complex (site this guys here). The X-ray crystal structure of a hypothetical 56 KDa Selenium Binding protein from Sulfolobus tokadaii has been solved but it has revealed a little about its function. (http://www.ncbi.nlm.nih.gov/Structure/mmdb/mmdbsrv.cgi?Dopt=s&uid=61601). The amino acid sequence has 472 amino acids, with the ORF of SeBP having 87% of homology to Acetaminophen Binding Protein (APBP). APBP is knwon to be present in the liver it's role is associated with hepatic necrosis in patients who have taken an acetaminophen overdose. ( Ishida, T. et al, 2002[ref 7]). Therefore here, we use structural comparisons,literature review and evolutionary analysis to try and infer the function of a 56 KDa Selenium Binding Protein.