Introduction 2ece
The trace element Selenium (Se) has been known to cause major health problems in livestock and man in soils which are rich in this mineral [Birringer, M., Pilawa, S. and Flohe, L. (2002]). Due to this association of selenium rich soils with these health problems, the beneficial role of this element was severely overlooked until the 1950's when it was discovered to be involved in the synthesis of most enzymes, like redox enzymes. The roles of selenium into important biochemical reactions was pioneered by Thressa Stadtman selenium biochemistry by the early 1970’s after an observation that bacterial cells required selenium, in their synthesis of an important enzyme, glycine reductase( History of selenium biochemistrySelenium biochemistry video movie file).Further research was carried out and it was found that this element can substitute sulphur in the cysteine amino acid and form a 21st amino acid dubbed as selenocysteine and coded UGA in amino acid codons. Selenium can as well be found in selenomethionine amino acid. Selenium exists in the form of selenate in soil and after absorption it undergoes a series of biochemical steps that involves a passage or binding of one protein to another. It is as well known that selenium in mammals has nutritional benefits and can be transported via haemoglobin and the mechanism and/or protein that mediate its transition from the blood to the whole areas in mammalian body where Selenium plays some important roles has not yet been clearly figured out (Haratake et al, 2008[[1]]) .
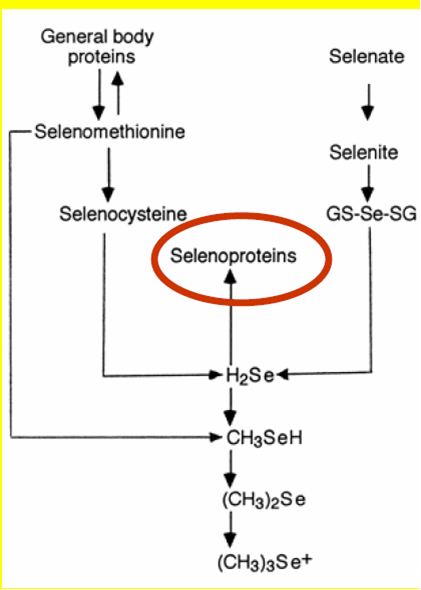
Adapted and modified from: Journal of nutrition 30: 1653 (2000) by Thiel File:TlectSel selenoproteins.pdf
Research has long since shifted from the toxicity of selenium and focused on its benefits as it was observed that it is an essential trace element required in human diet and it can be incorporated into amino acids. People with selenium deficiency were observed to be prone to cancer, the root cause being the fact that selenium is a cofactor in enzymes that are involved in hydroperoxide detoxification and other anti-carcinogenic activities. However, further mutational studies have revealed that this issue is more complex (site this guys here). The X-ray crystal structure of a hypothetical 56 KDa Selenium Binding protein from Sulfolobus tokadaii has been solved but it has revealed a little about its function. (http://www.ncbi.nlm.nih.gov/Structure/mmdb/mmdbsrv.cgi?Dopt=s&uid=61601). Therefore here, we use structural comparisons and evolutionary analysis to try and infer the function of a 56 KDa Selenium Binding Protein.