Phytanoyl-CoA dioxygenase Structure: Difference between revisions
No edit summary |
No edit summary |
||
| Line 3: | Line 3: | ||
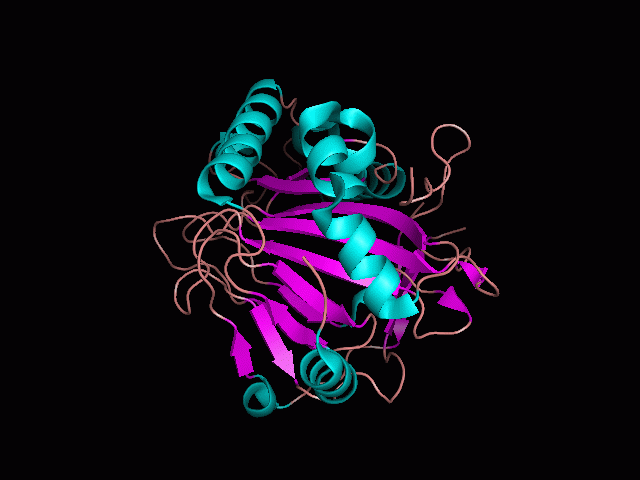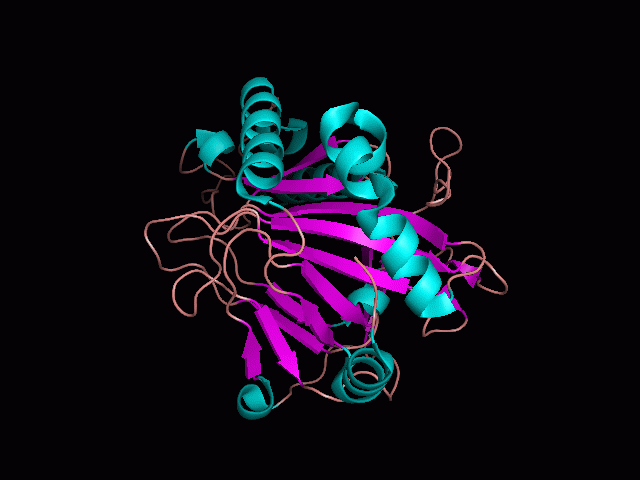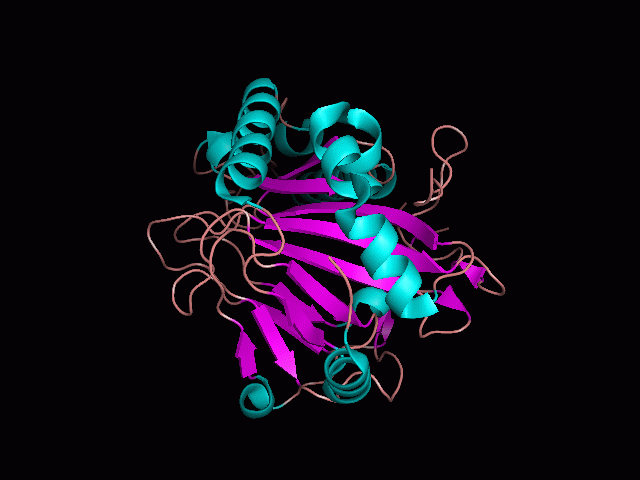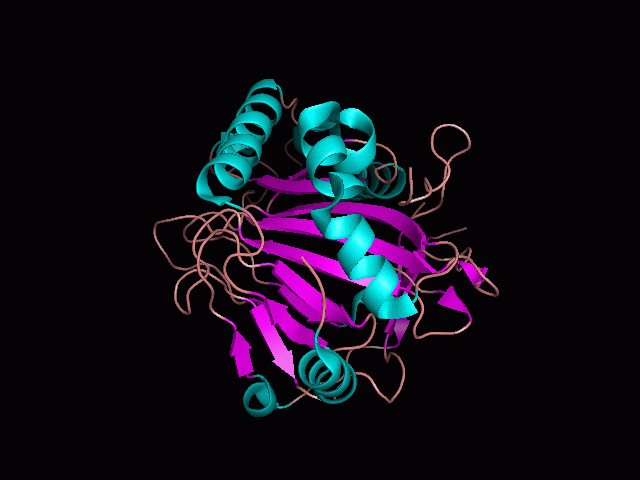
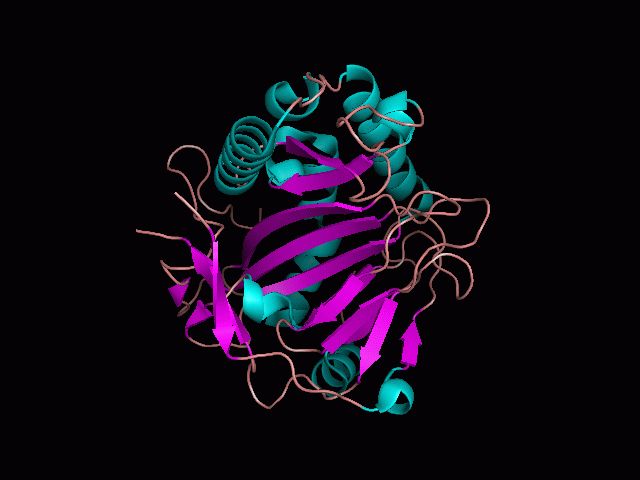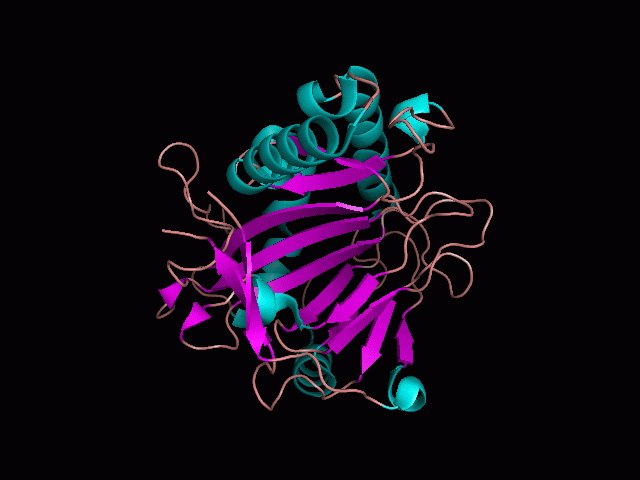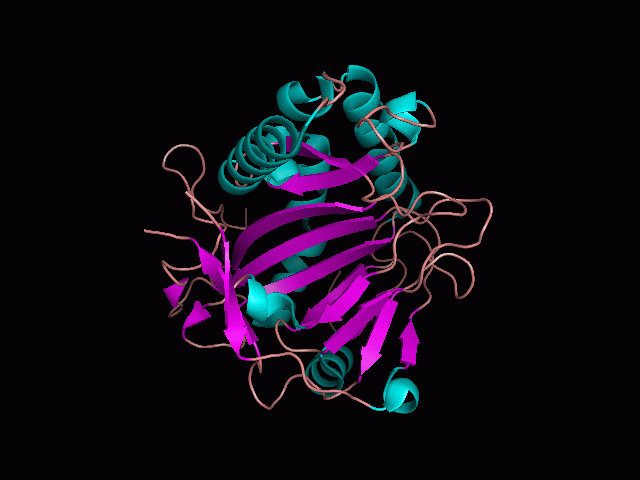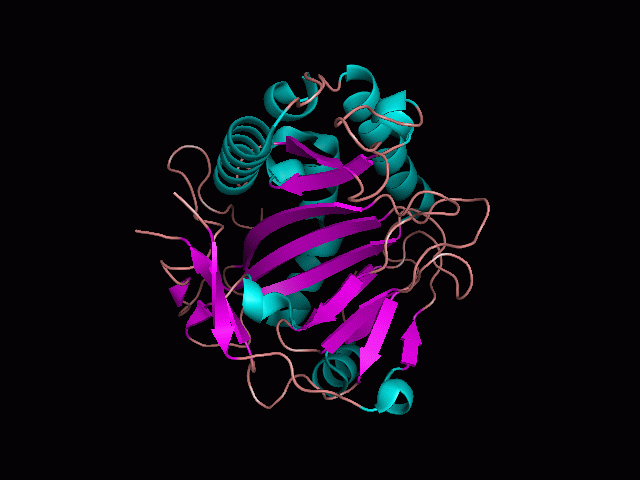
Originally, the structure of our protein was experimentally defined (by Zhang, Z., Butler, D., McDonough, M.A et.al.) via X-ray diffraction. | Originally, the structure of our protein was experimentally defined (by Zhang, Z., Butler, D., McDonough, M.A et.al.) via X-ray diffraction. | ||
<br><b> Name </b> | <br><b> Name - </b>Phytanoyl-CoA dioxygenase (PHYHD1) | ||
<br><b> Classification - </b> Oxidoreductase | |||
<br><b> Resolution (Amstrongs) - </b> 1.90 | <br><b> Resolution (Amstrongs) - </b> 1.90 | ||
<br><b> R-Value - </b> 0.221 (obs, relatively low) | <br><b> R-Value - </b> 0.221 (obs, relatively low) | ||
Revision as of 07:17, 8 June 2008
Originally, the structure of our protein was experimentally defined (by Zhang, Z., Butler, D., McDonough, M.A et.al.) via X-ray diffraction.
Name - Phytanoyl-CoA dioxygenase (PHYHD1)
Classification - Oxidoreductase
Resolution (Amstrongs) - 1.90
R-Value - 0.221 (obs, relatively low)
Space Group - P 3.1 2 1
Unit Cell Paramters (Amstrongs) - a = 91.97, b = 91.97, c = 81.61
Unit Cell Angles - alpha = 90.00, beta = 90.00, gamma = 120.00
There are high levels of steric hinderance if the approaching ligand comes from behind the molecule (figure 1).
There is a large hole in the structure of our protein (figure 2). As steric hinderance is low and the surface area is maximized at this position, it would seem that this area should encompass the binding sites of our protein, or at least be the location of some form of interaction. The fact such a definitive hole in the structure of the protein exists lead us to investigate further properties around this region.
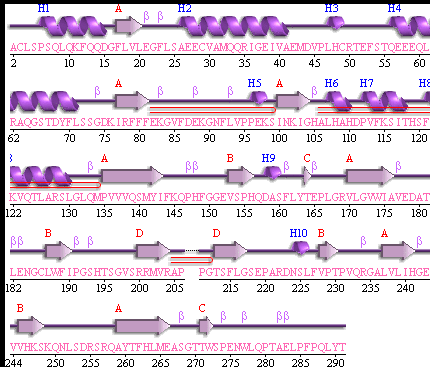
Showing the topology of the protein. Reproduced from the European Bioinformatics Institute,
http://www.ebi.ac.uk/thornton-srv/databases/pdbsum/2opw/domA01.gif
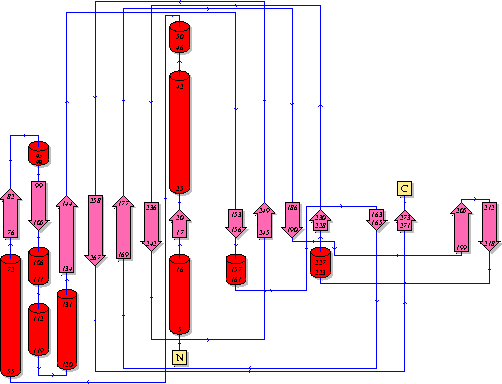
Showing another topology of the protein. Reproduced from the European Bioinformatics Institute,
http://www.ebi.ac.uk/