Results ERp18: Difference between revisions
No edit summary |
No edit summary |
||
| Line 17: | Line 17: | ||
Both Pfam and InterPro identified the protein as the Middle domain of eIF4G, termed MIF4G. MIF4G consists essentially of alpha helices and has “multiple alpha-helical repeats” | Both Pfam and InterPro identified the protein as the Middle domain of eIF4G, termed MIF4G. MIF4G consists essentially of alpha helices and has “multiple alpha-helical repeats”. Within eIF4G, it binds to the RNA helicase eIF4A, eIF3, RNA and DNA. | ||
The DALI server was used to identify proteins with similar structure to MIF4G-like protein from Danio rerio (PDB:2i2o). From the hits generated, three proteins (PDB:1hu3, 1uw4, 1h6k) with Z-scores 15.2, 10.9 and 10.6 respectively were selected. 1hu3 is the middle domain of human “Eukaryotic Translation Initiation Factor 4G (eIF4G)”. 1uw4 is an mRNA decay factor and 1h6k is the human nuclear cap binding protein complex (CBC). | |||
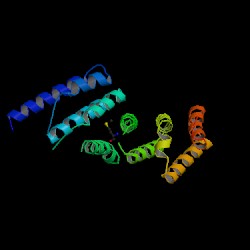
A CE structural comparison of 1hu3 with the zebrafish putative MIF4G revealed much similarity in folding and protein component. 4 alpha-helices folded in the same orientation can be identified on each protein. The generated figure shows a superimposed image of both proteins, hence suggesting an overall similarity in structure. The N-terminal of 1h6k is highly similar in fold and orientation as MIF4G. This implies a possibility that MIF4G protein is a region, or even an active domain, near the N-terminal of the CBC. | |||
Revision as of 04:48, 5 June 2007
Evoltuion of MIF4G Domain Containing Protein
Results notes
First identified in Dario rerio (zebra fish)
Eukaryotic gene - derived gene
Protein conserved throughout the Metazoans (animals)
Structure of MIF4G Domain Containing Protein
From the PDB search, the structure was revealed to be similar to “Eukaryotic Translation Initiation Factor 4G (eIF4G)” protein. The protein was isolated form Danio rerio and expressed in Escherichia coli. It is formed from two chains, with two chemical components, nickel (Ni2+) and selenomethionine (C5H11NO2Se) as additions to the protein. The NCBI Entrez search revealed the protein to be a domain of the eIF4G-like protein.
Both Pfam and InterPro identified the protein as the Middle domain of eIF4G, termed MIF4G. MIF4G consists essentially of alpha helices and has “multiple alpha-helical repeats”. Within eIF4G, it binds to the RNA helicase eIF4A, eIF3, RNA and DNA.
The DALI server was used to identify proteins with similar structure to MIF4G-like protein from Danio rerio (PDB:2i2o). From the hits generated, three proteins (PDB:1hu3, 1uw4, 1h6k) with Z-scores 15.2, 10.9 and 10.6 respectively were selected. 1hu3 is the middle domain of human “Eukaryotic Translation Initiation Factor 4G (eIF4G)”. 1uw4 is an mRNA decay factor and 1h6k is the human nuclear cap binding protein complex (CBC).
A CE structural comparison of 1hu3 with the zebrafish putative MIF4G revealed much similarity in folding and protein component. 4 alpha-helices folded in the same orientation can be identified on each protein. The generated figure shows a superimposed image of both proteins, hence suggesting an overall similarity in structure. The N-terminal of 1h6k is highly similar in fold and orientation as MIF4G. This implies a possibility that MIF4G protein is a region, or even an active domain, near the N-terminal of the CBC.