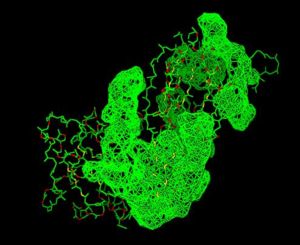
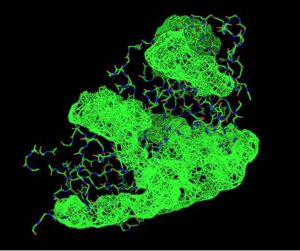
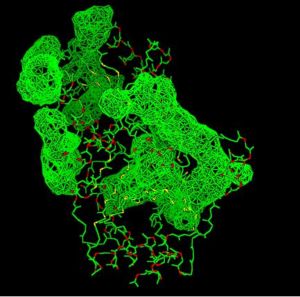
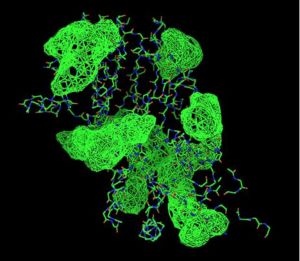
Pyridoxal Phosphatase Results
Evolution
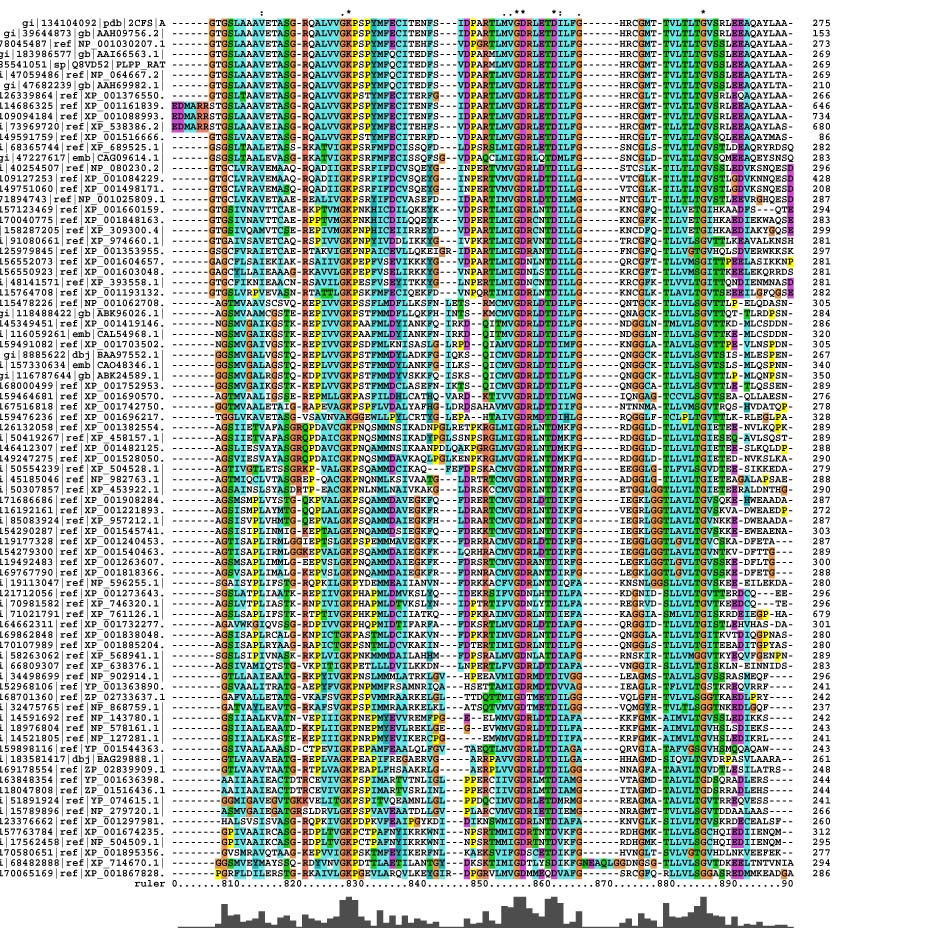
Multiple Sequence Alignment
Pyridoxal Phosphatase Multiple Sequence Alignment
Structure
General Structure of Pyridoxal Phosphatase

PDB
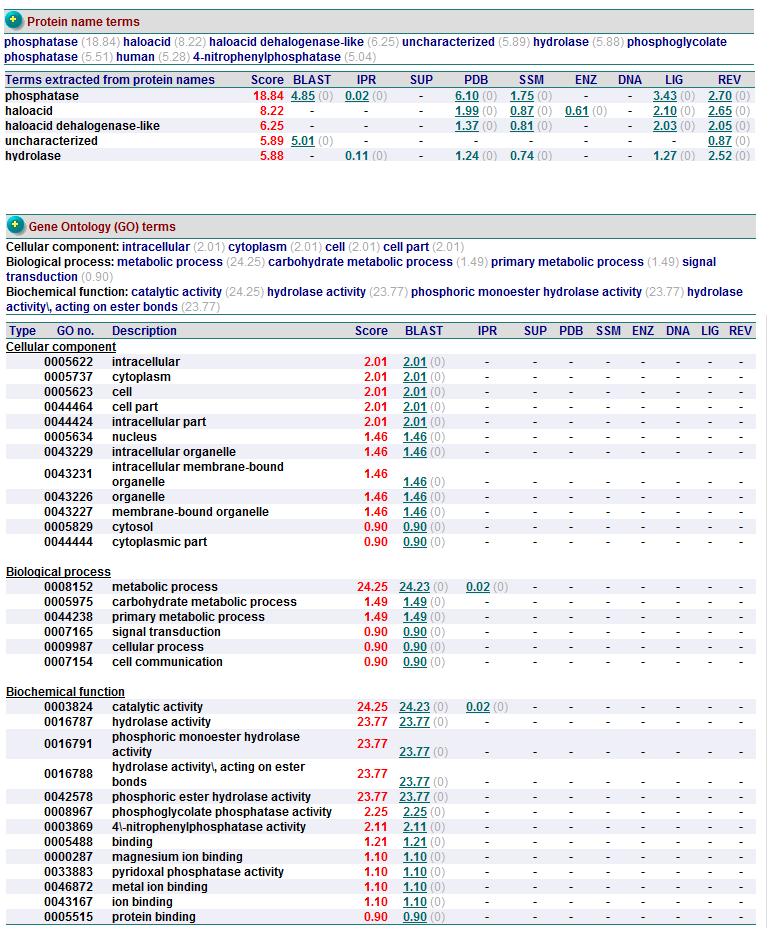
Based on the information provided in the website, Pyridoxal Phosphatase has the following characteristics:
- Pyridoxal Phosphatase is isolated from Homo Sapiens and is expressed in Escherichia Coli.
- Structure is similar to the Pyridoxal Phosphate Phospatase protein
- 1 (A) Chain
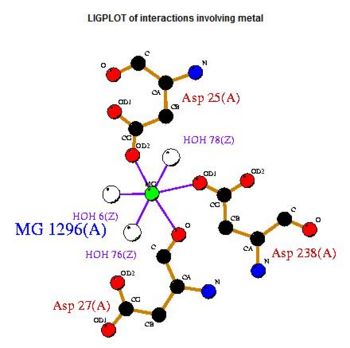
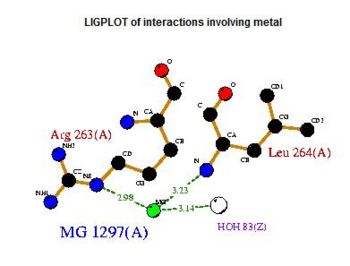
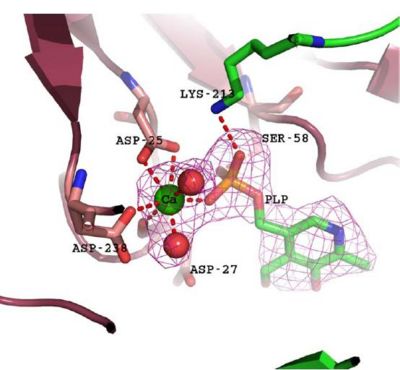
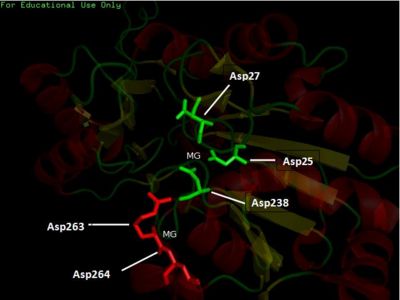
- Consists of Magnesium components
- Resolution of 2.40 angstroms. This means that the number of sidechains in the wrong rotamer is smaller as compared to proteins of a higher resolution (>2.5 angstroms). Other characteristics of proteins of similar resolutions are: (1) many small detectable errors, (2) correct folding, (3) fewer number of errors in the surface loops and (4) visible water molecules and small ligands.
http://www.rcsb.org/pdb/explore/explore.do?structureId=2cfs
DALI
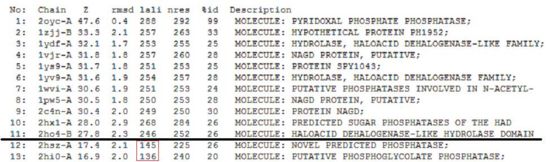
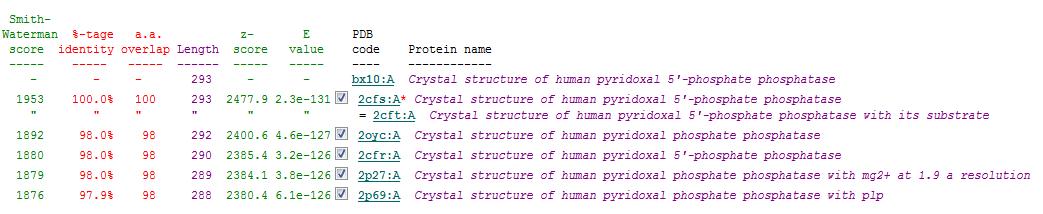
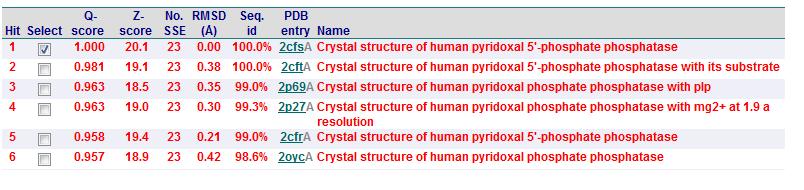
A total of 176 hits were generated, of which only the first 11 (as shown in Fig. 3) were predicted to be significant. The others were rejected on account that their respective lali scores (refer to the red, boxed section) were less than half of Pyridoxal Phosphatase's (nres: 296). The lali value is important as this refers to the number of structurally equivalent residues between the hit protein and the protein-of-interest. If two proteins are not structurally similar/related, chances are their functions will not share similarities.
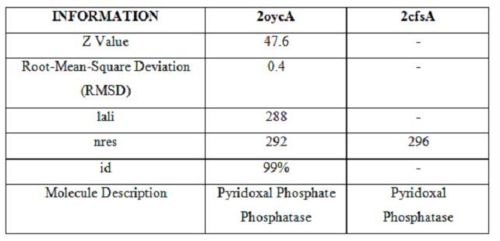
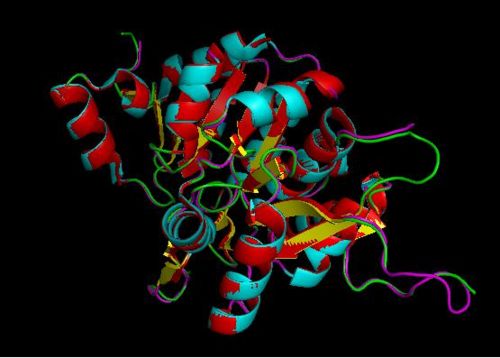
It was noted that none of the hits actually matched 2cfsA. The closest was a Pyridoxal Phosphate Phosphatase (PDB ID 2oycA) which based on the results, was predicted to be highly similar to 2cfsA. Table 1 highlights the similarities between Pyridoxal Phosphatase (2cfsA) and the Pyridoxal Phosphate Phosphatase (2oycA)
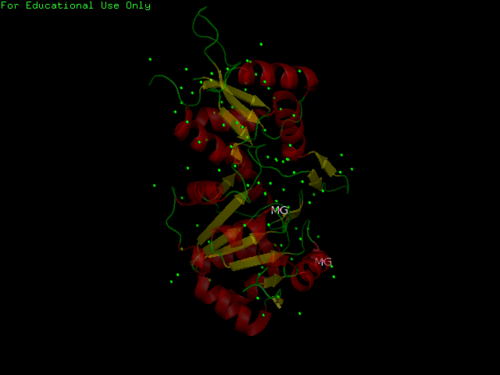
Using the PyMOL software, 2oycA was superimposed against 2cfsA, and both structures, as shown in Fig. 4, are structurally similar.
PDBsum
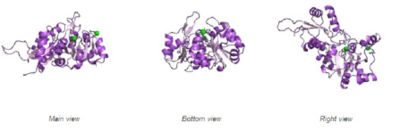
Fig. 5 offers three different views of 2cfsA. The purple chains represent the amino acid chain of 293 amino acids while the green spheres represent the magnesium ions (x2).
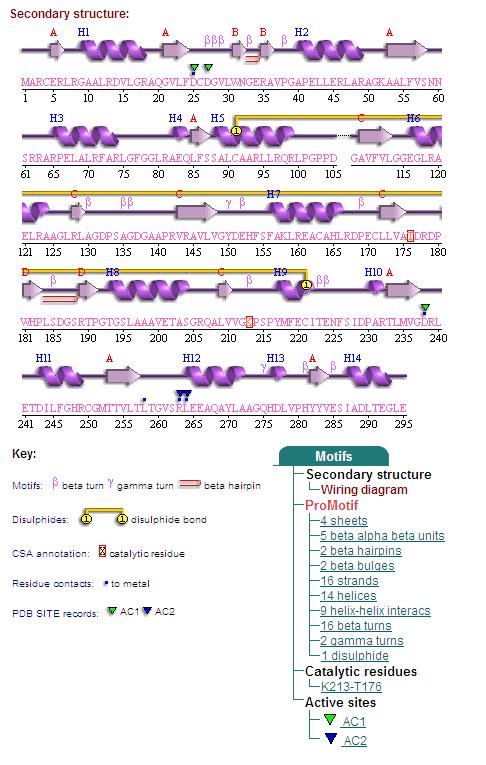
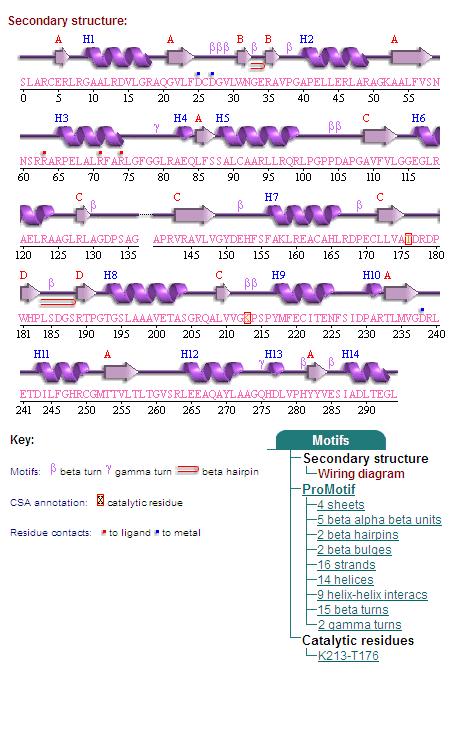
By clicking the "Protein chain" link, the user was re-directed to a website containing information pertaining to the secondary structures of both 2cfsA and 2oycA.
Secondary Structures of 2cfsA (L, http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl) and 2oycA (R).
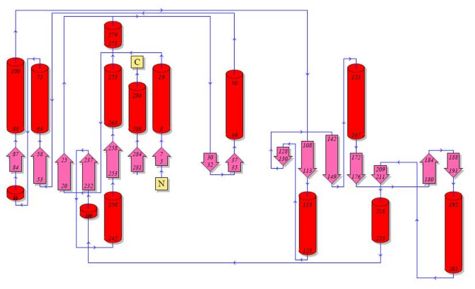
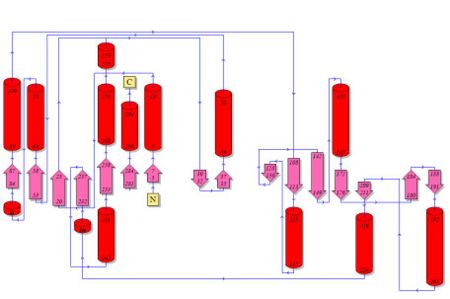
Topology diagrams of 2cfsA (Fig 6.1. http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=2cfs&template=protein.html&r=wiring&l=1&chain=A) and 2oycA (Fig 6.2). The topology diagram is a simplified version of the secondary structure information as provided above, and is an indication of the location of the alpha helices (represented by the red cylinders) and beta strands (represented by the pink arrows).
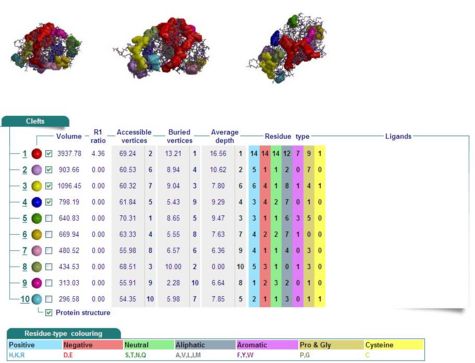
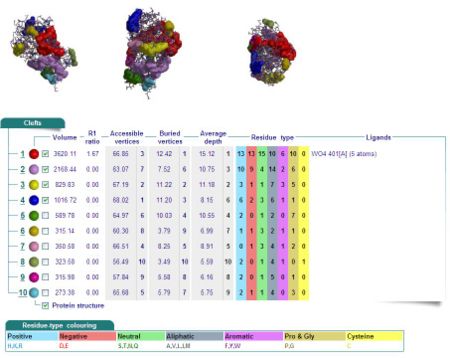
Cleft Analyses of 2cfsA (Fig 7.1. http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=2cfs&template=clefts.html&pdbcode=2cfs&r=speedfill) and 2oycA (Fig 7.2).
Cleft Analysis via PyMol
PROFUNC
Related Protein Sequences in the PDB (SAS)

Matches to existing PDB Structures
Secondary Structure Matching (SSM)
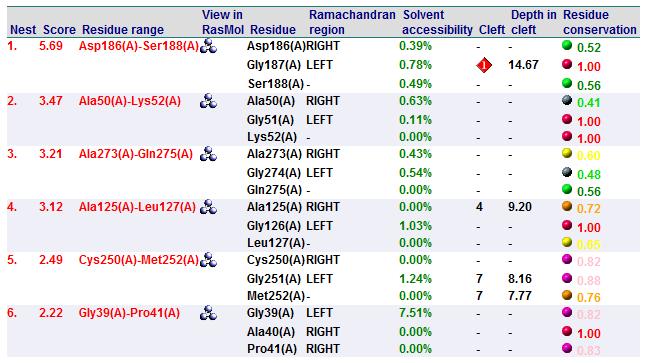
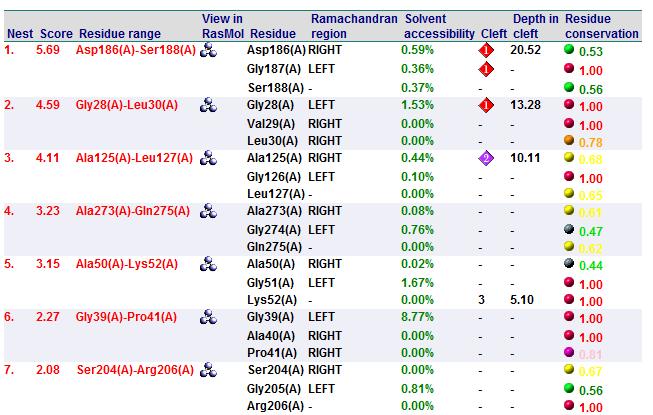
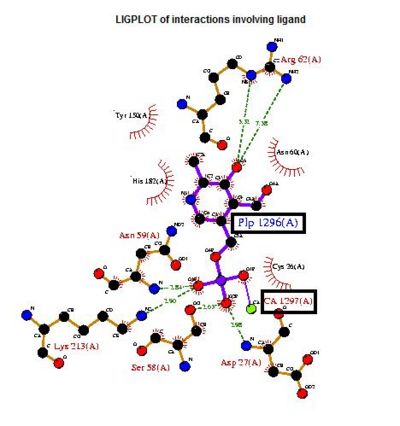
A search on 2oycA was also carried out via PROFUNC, and consistency in the results confirmed that 2cfsA and 2oycA were indeed structurally similar. This lends weight to the original hypothesis that they could be functionally related. In fact, based on the Nest Analysis method, 2oycA was observed to be sharing a large number of active sites. The results of the Nest Analysis (2oycA) are as shown below. Notice the similarities between the active sites of both 2oycA and 2cfsA.
In addition, the predicted function of 2oycA was similar to 2cfsA.
Based on the above-mentioned results, it was concluded that 2cfsA, 2cftA and 2oycA were structurally similar, and that further functional studies could indeed uncover the function of the protein-of-interest: 2cfsA