C1orf41 Results
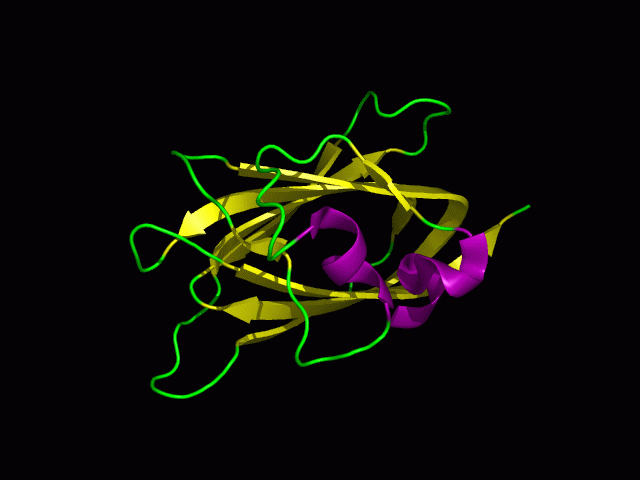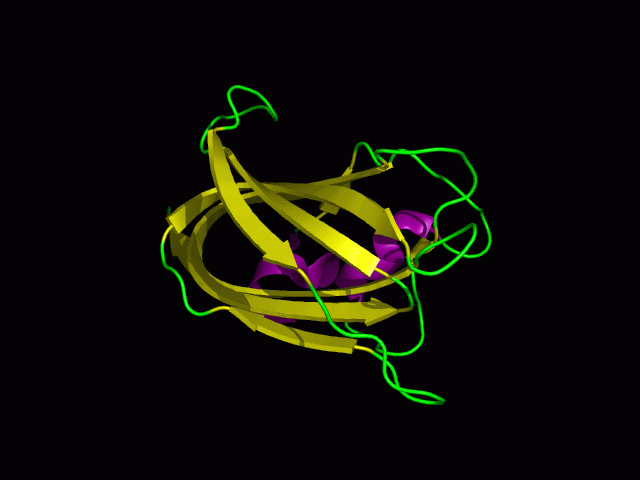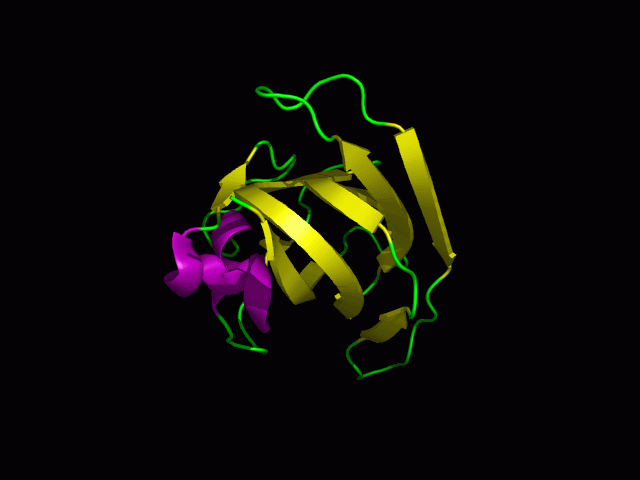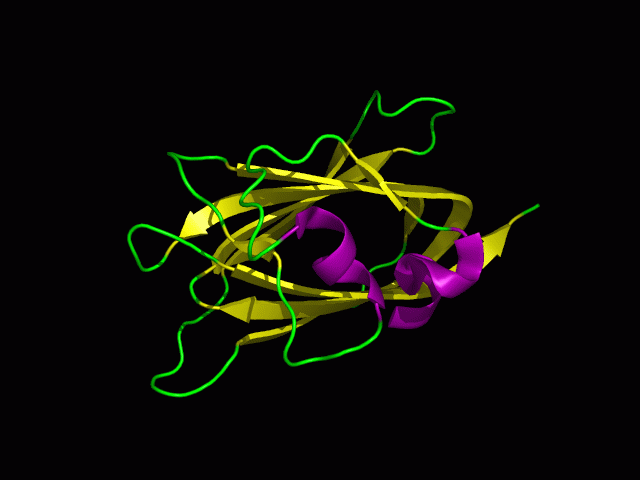
Structure
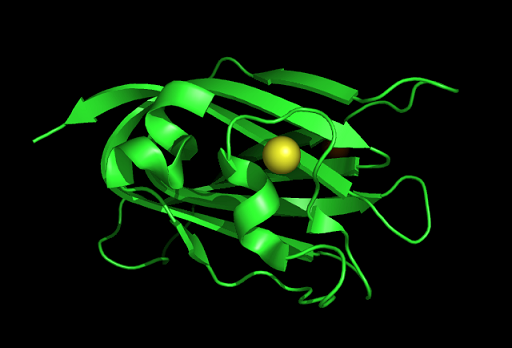
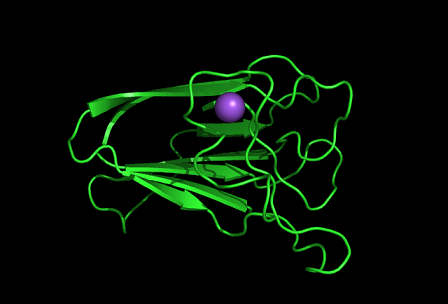
PDB ID: 1TVG Information from the PDB stated that x-ray diffraction was ueed to solve the structure of this protein with an R value of 0.215 and at 1.6Å. Two ligands were present in the crystal structure, a calcium (II) ion and a samarium (III) ion. This protein has 153 residues and its secondary structure consists of two helices and eight beta strands.
Scop result
Tertiary structure
Structural analysis
1)Structural similarities
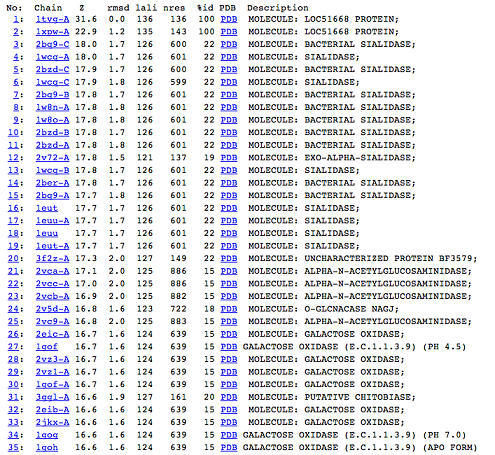
DALI was used to find proteins that are similar in structure to c1orf41. The results showed that sialidases, alpha-N-acetylglucosaminidases and galactose oxidases have similar structures to this protein.
2)Domain Classification
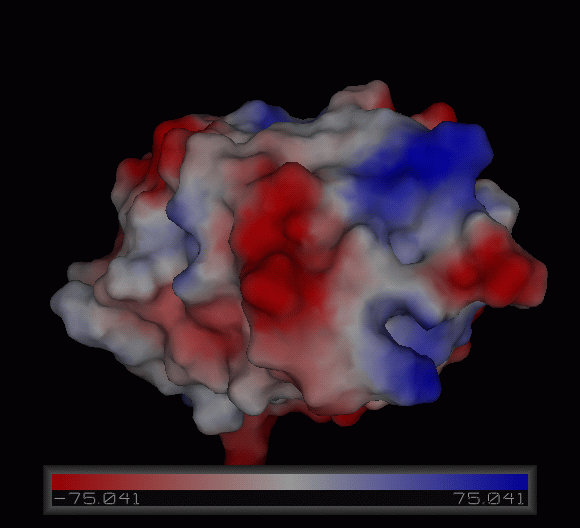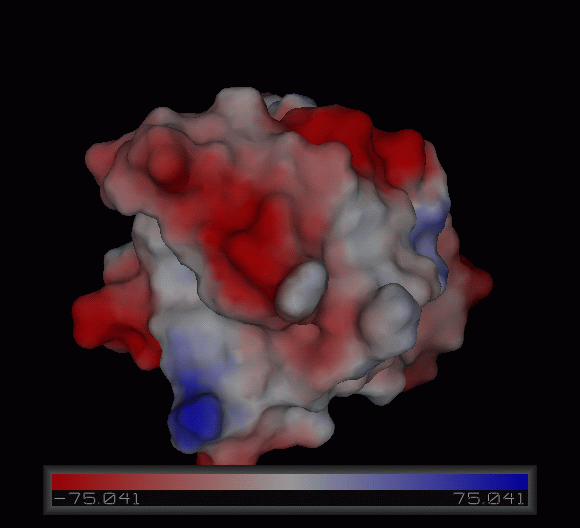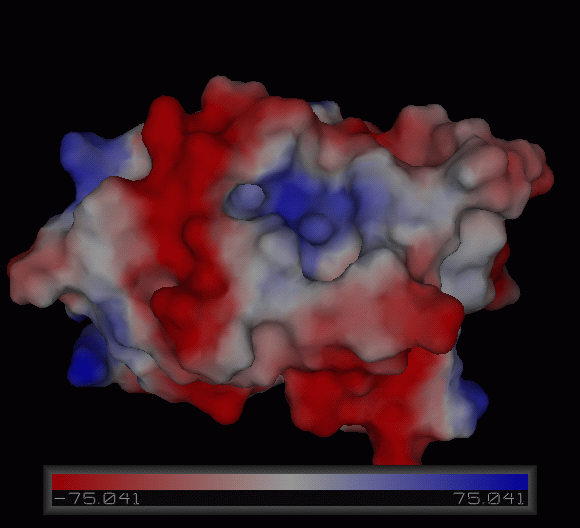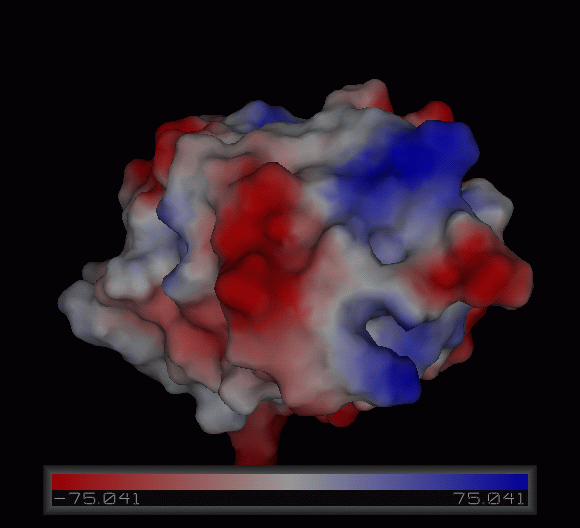
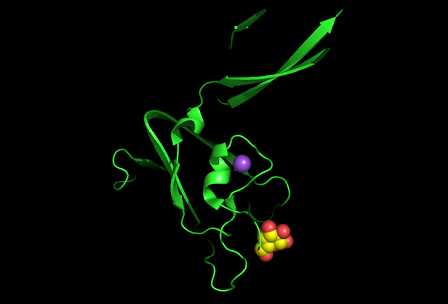
3)Possible ligand binding sites
Observations and comparisons of several proteins from the DALI result with our protein showed that there is a similar position on each proteins where a metal ion is located.
Nest analysis by Profunc suggested two other ligand binding sites. Nests are structural motifs that are commonly found in functionally important regions of protein structures.
Evolution
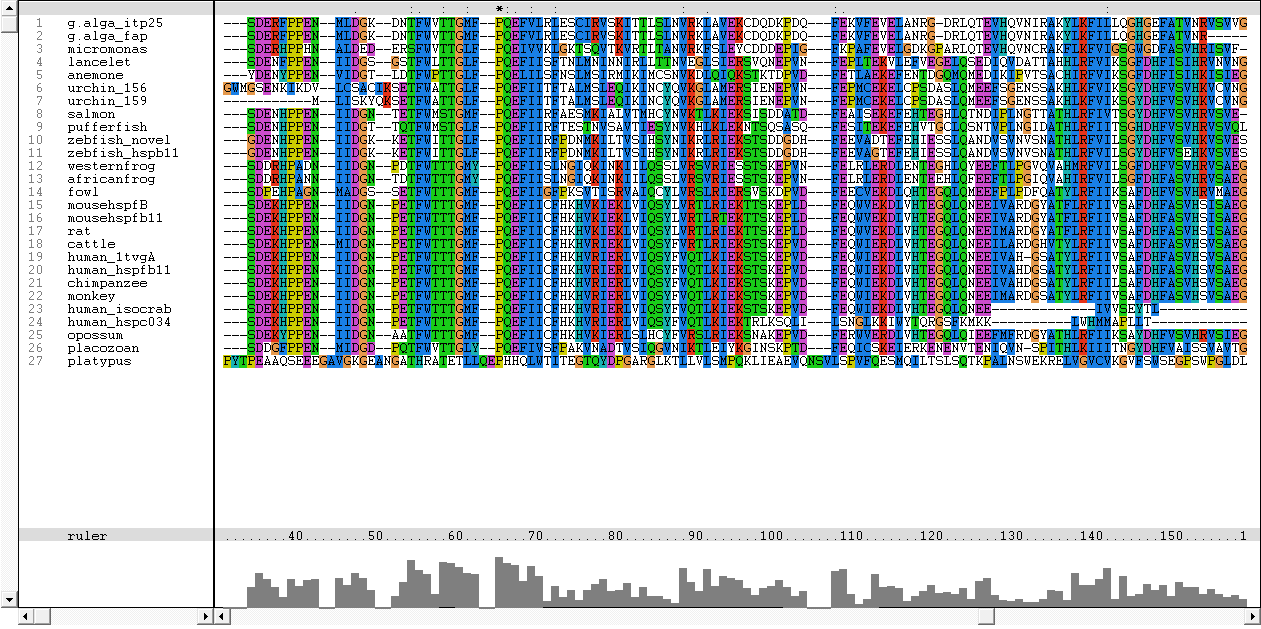
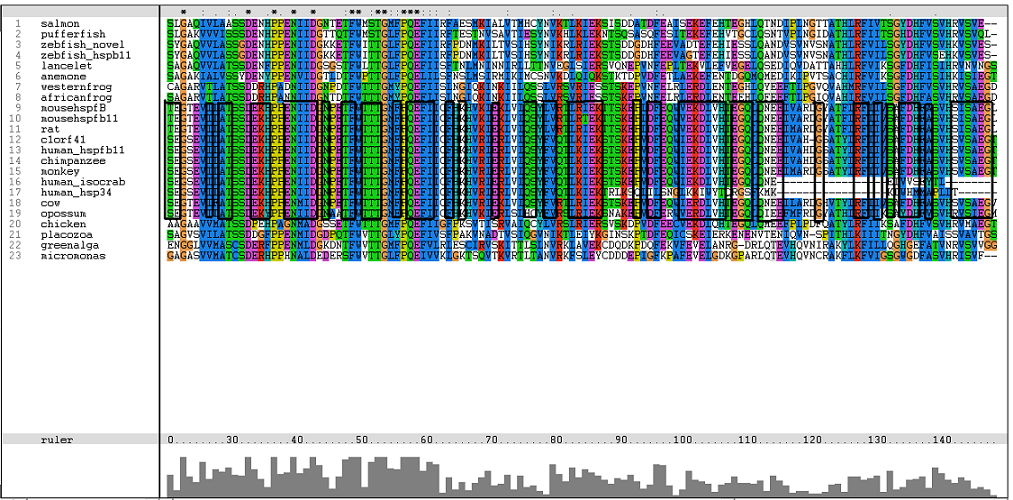
Results from Blast showed that c1orf41 have high homology to small heat shock proteins which gave us a hint that the protein may be a heat shock protein (Figure 14). However, initial multiple sequence alignment showed many gaps and few conserved regions (Figure 15).
The sequences from platypus, urchin_156, urchin_159 and g.alga_fap were excluded from further investigation. This was due to poor alignment of platypus, urchin_156 and urchin_159 with the other organisms. In addition, the sequence for g.alga_fap was removed as it was a truncated sequence of g.alga_itp25.
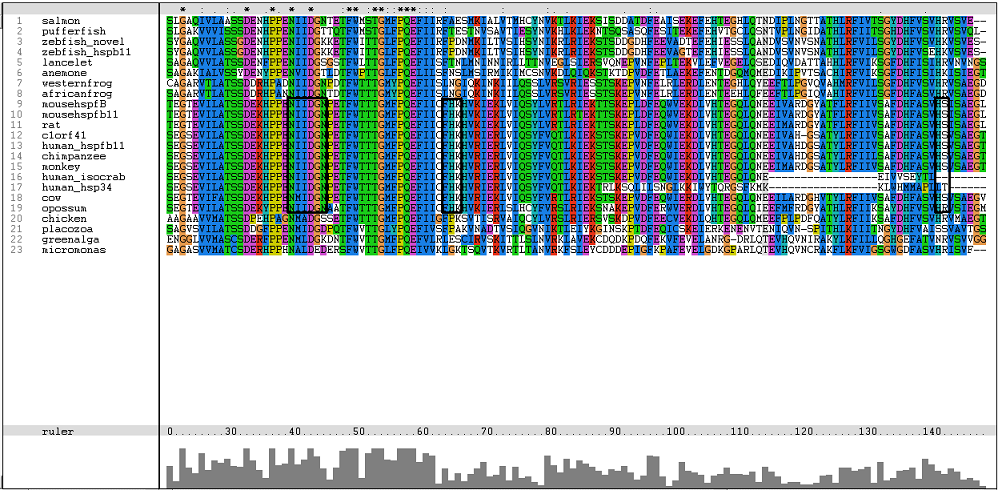
One of the characteristic of a small heat shock protein is that they have a conserved alpha B crystallin domain which can also be observed in c1orf41 as shown in figure 16 below:
The conserved residues found in c1orf41 and its homologues fall in the alpha B crystallin domain. But more conserved regions are observed in higher terrestrial eukaryotes. With this, we are more confident that c1orf41 is a heat shock protein.
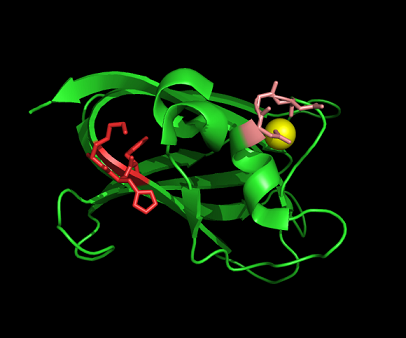
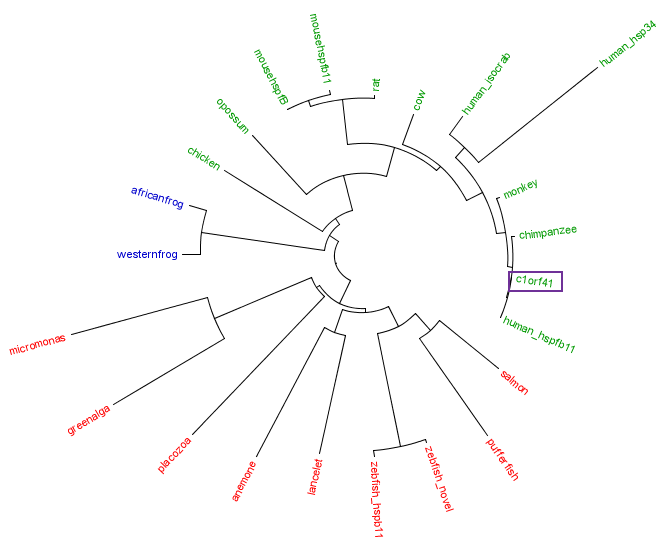
The residues of the possible binding site were highlighted in black in figure 17 below. However, these residues were only conserved in terrestrial eukaryotes. During evolution, the protein has evolve and adopt different function in marine eukaryotes, amphibian and terrestrial eukaryotes. The evolutionary relationship of this protein is shown in figure 18.
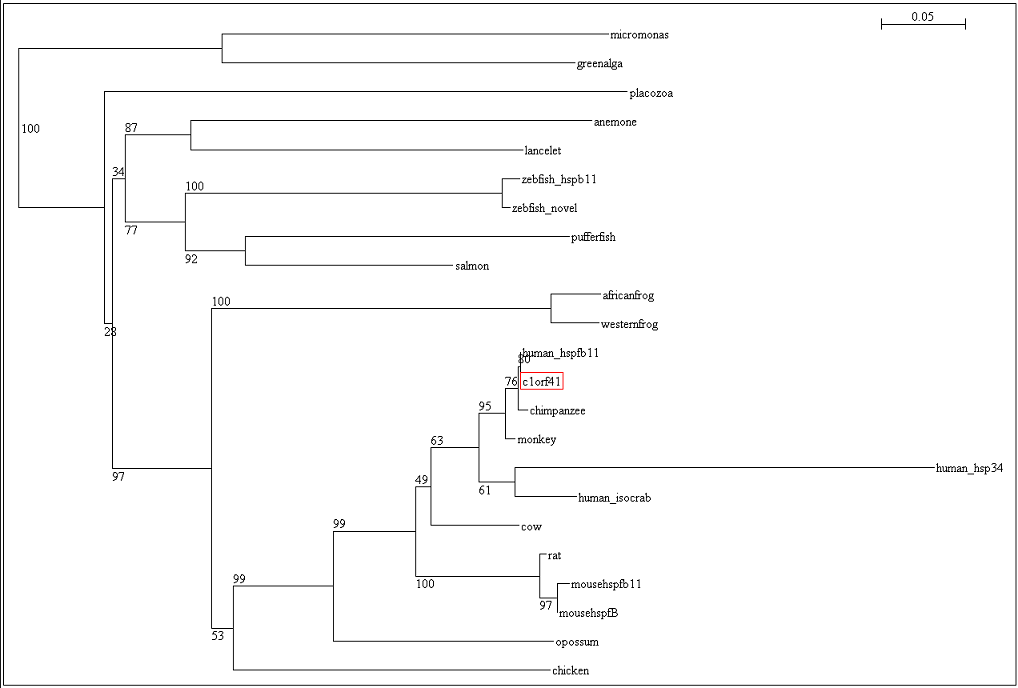
To ensure that the reliability of the evolutionary relationship of c1orf41 and its homologues, the tree was bootstrap with 100 replicates (Figure 19). Bootstrap value of more than 75 indicated that we are confident in the branch whereas bootstrap value of less than 50 indicated no confidence in the branch over an alternative
Function
Information from sequence analysis (i.e. Blast, structure analysis (i.e.domain classification (Pfam) and possible ligand binding site) was used to infer the function of c1orf41. Profunc server was also run to get a summary of sequence and structural analysis. Relevant information was used to analyze the function.
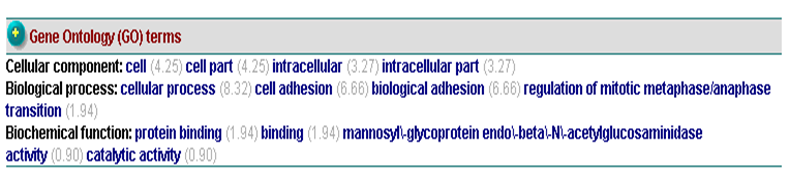
Gene ontology terms suggested that c1orf41 involves in cellular process or cell adhesion (Figure 20).
Based on Blast result, our protein has high similarity with sHsp. Thus, they may have similar function. sHsp functions as molecular chaperone and cytotprotective (antiapoptotic). The C1orf41 has a loop binds to Ca2+ which presumably related to antiapoptotic function of sHsp.
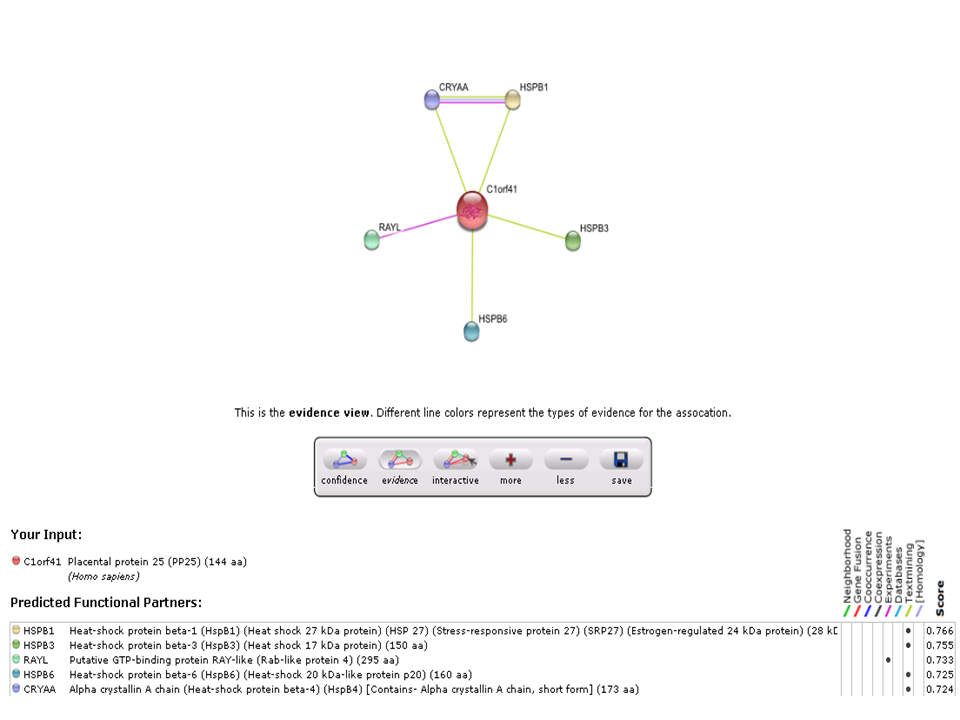
The String analysis showed protein-protein interactions of c1orf41. Based on experimental evidence, there was an interaction between c1orf41 and RAYL (Ras-like-GTPase superfamily member). Overexpression of Ras GTPase activating protein will enhance the phosphorylation & activity of Akt which leads to antiapoptotic activity.
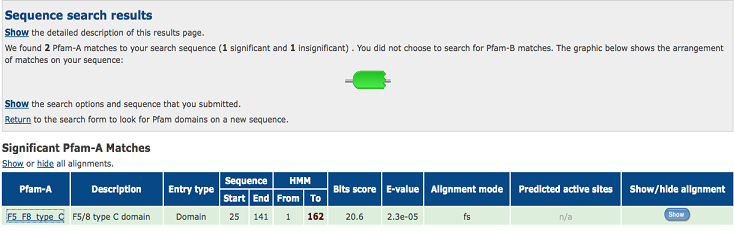
Discoidin
Based on Pfam result (Fig.6), our protein target is a member of F5/8 type C (discoidin)domain. It is reported that discodin is involved in cell-cell interaction & recognition and also highly express in breast carcinoma cell lines. However, discoidin is extracellular protein, while c1orf41 is intracellular. Moreover, based on sequence and structural analyses, our protein is more likely a sHsp.