Hypothetical protein Introduction
Breif Introduction
Early literature review suggests that our protein is either RbsD or FucU, it is thought that both are of similar function. FucU may play a similar role as RbsD by binding L-fucose (Kim et al. 2003). It is tentatively suggested that these two proteins are involved in the binding, transport or possibly metabolism of sugar molecules in cells. Their involvement in any one of these processes is not yet clear or understood. It is thought RbsD and FucU play a role in the d-ribose and L-fucose transport respectively. D-ribose and L-fucose are forms of sugars and are used as energy sources. In the sequence analysis, RbsD shows no predicted transmembrane domain and exhibits sequence similarity to RbsD homologues in other organisms and to FucU, which is a component of the fucose operon (Kim et al 2001). Further research on these proteins has however identified possible biochemical functions. It seems that these two proteins are involved in the initial binding of the sugar molecules. Kim et al. (2003) demonstrated that the biochemical function of RbsD and FucU is to bind specific forms of D-ribose and fucose, respectively. This was concluded through the identification of conserved residues at the sugar binding sites. Kim et al (2003) still has reservations about the exact function suggesting that the proteins play a role in facilitating the influx of the sugar substrates.
Introduction
Cells require material and information from their surrounding environment through which they can grow and life can develop. Time and evolution have thus allowed for the development of effective and efficient transport systems through which all types of cells gain and/or displace the appropriate material and information that is essential for growth and survival. These systems ensure essential ions and metabolites enter the cell and other compounds leave it (Jasinski et al. 2003). Binding protein-dependent ATP-binding cassette (ABC)-type systems have been found to mediate the uptake of solutes such as amino acids, peptides, ions, vitamins, and sugars in a wide range of bacteria (Tuner et al. 1999).These ABC transport systems are found in all forms of life, both eukaryotes and prokaryotes. Our protein is part of the superfamily RbsD/FucU and plays a role in one of these ABC transport systems. Its specific role in these systems is yet to be confirmed.
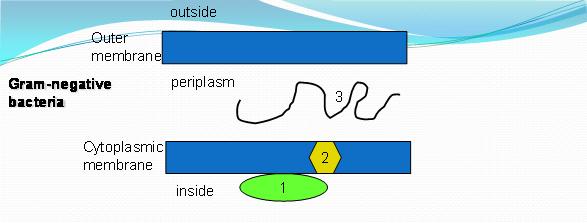
ABC proteins have two basic structural elements that can be found in both prokaryotes and eukaryotes. These two elements include: a hydrophobic transmembrane domain (TMD) usually made of six membrane-spanning-helices, and a cytosolic domain, the latter containing a region involved in ATP binding and known as the nucleotide-binding domain (NBD) (Jasinski et al. 2003). This being said the superfamily of transport systems can be divided into two kinds of proteins: prokaryotic type (PK-type) and the eukaryotic type (EK-type). The PK-type is distinguished from the EK-type by gene and domain organisation. The structure of a typical PK-type ABC transporter consists of three types of components: (1) two integral proteins each having six transmembrane segments, (2) two peripheral proteins that bind and hydrolyze ATP, and (3) a periplasmic substrate binding protein (Igarashi et al 2004) refer to figure 1. The difference with the EK-type is that they do not contain the substrate binding protein.
It is thought that our protein 2ob5A is either RbsD or FucU, as indicated by it being part of the joint superfamily of RbsD/FucU. The reason for this is that FucU and RbsD play similar roles in these ABC systems (Kim et al. 2003). The difference between the two is the solute that is bound, transported and metabolised in the system. The solutes in question are two sugars, fucose and ribose, more specifically d-ribose and l-fucose: which are forms of primary sugars. For these sugars, or any solute for that matter, to pass through the cytoplasmic membrane and into the cell, they have to be either bound to the specific solute-binding protein or be of the correct form to pass through the solute channel. For this to take place some solutes are required to go through certain changes to be able to bind or pass through the cytoplasmic membrane. It seems that these two proteins, RbsD and FucU, are involved in the initial binding of the sugar molecules. Kim et al. (2003) demonstrated that the biochemical function of RbsD and FucU is to bind specific forms of D-ribose and fucose, respectively. This was concluded through the identification of conserved residues at the sugar binding sites. They alter the form of their respective sugars allowing for them to bind and allow the solute transport to continue. Kim et al (2003) still has reservations about the exact function suggesting that the proteins play a role in facilitating the influx of the sugar substrates.
The aim of this report is to provide viable information on the protein 2ob5A. The information provide will cover evolutionary history of the protein through the construction of a phylogenic tree, as well as provide insight into its structure and function through the use of numerous bioinformatics techniques. The information provided will hopefully assist in future research of this protein and related proteins.
Abstract | Introduction | Method |
Results | Discussion | Conclusion | References