Hypothetical protein Results: Difference between revisions
| (49 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
=='''Evolution'''== | =='''Evolution'''== | ||
Sequences gathered for MSA (including query): | ===Sequences=== | ||
Sequences (FASTA format) gathered for MSA (including query), available via the following link: | |||
[http://compbio.chemistry.uq.edu.au/mediawiki/index.php/Image:Fewerseqforalign.txt] | [http://compbio.chemistry.uq.edu.au/mediawiki/index.php/Image:Fewerseqforalign.txt] | ||
----------- | |||
===Multiple Sequence Alignment=== | |||
[[Image:hypotheticalmsa1xx.jpg]] | |||
Multiple sequence alignment generated using Clustalx 1.83 [1]. | |||
------------- | |||
===Original Curved Bootstrapped Tree=== | |||
[[Image:hypotreexx21.jpg]] | |||
Curved phylogenetic tree with bootstrap values, obtained using MEGA 4.0 [2] | |||
---------------- | |||
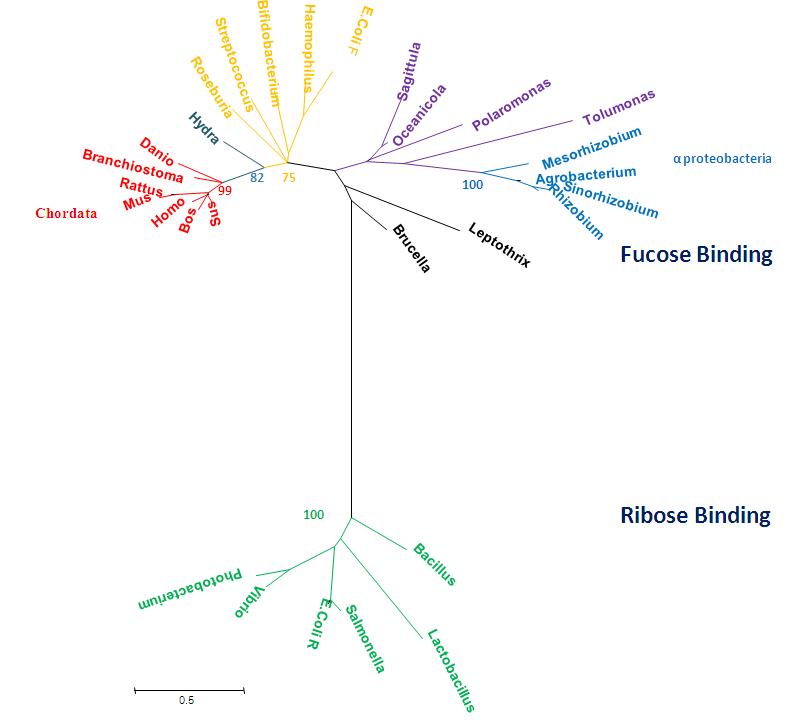
===Radiation Tree showing relevant bootstrap values=== | |||
[[Image:radiationtreexx22.jpg]] | |||
Phylogenetic tree representing radiation, obtained using MEGA 4.0 [2] and manually edited in Microsoft Power Point 2007. | |||
=='''Structure'''== | |||
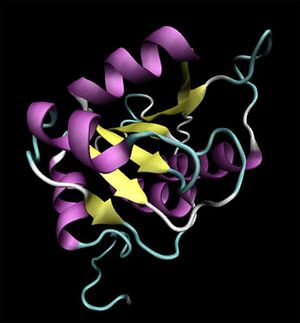
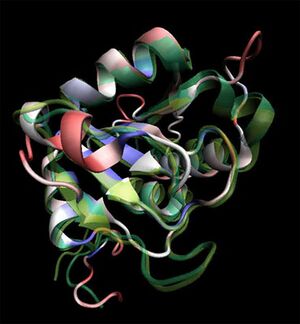
[[Image:2ob5_main.jpg|left|thumbnail|Figure 1. Crystal structure of 2ob5 showing secondary structural elements]] | |||
==Crystal structure== | |||
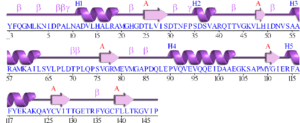
[[Image:2ob5_sec_struct.gif|right|thumbnail|Figure 2. Schematic of 2ob5 secondary structure]] | |||
Initial structural analyses indicate that 2ob5A is a member of the RbsD/FucU protein superfamily (Sanger Institute 2001), and is of the RbsD-like fold classification (Murzin et al. 1995). It is a globular protein composed of five alpha-helixes arranged around six internal beta strands in the tri-layered a/b/a fold characteristic of RbsD-like proteins (EMBL-EBI PDBSum 2007) (Figure 2). | |||
==Structural alignment searches== | |||
===Dali search=== | |||
[[Image:1ogd_3e7n_main.jpg|left|thumbnail|Figure 3. Crystal structures of 1ogd (A) and 3e7n (B)]] | |||
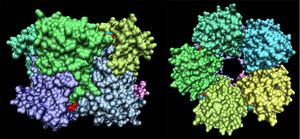
Early structural alignments (Holm & Sander 1993) supported this classification, returning ribose-transport proteins as high similarity hits. Of the structures found, the majority of high-significance (z > 15) matches were variations on two prokaryotic RbsD proteins, 3e7n (D-ribose high affinity transport system, S. Typhi) and 1ogD (high affinity ribose transporting protein, B. Subtillis). These two crystal structures are shown in figure 3 (RCSB 2008). | |||
===Structures of matching proteins=== | |||
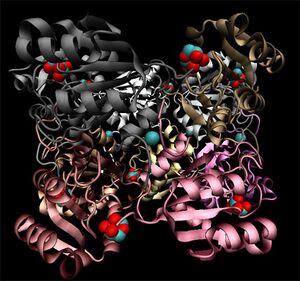
[[Image:3e7n_complex_surf.jpg|right|thumbnail|Figure 4. Quaternary structure of 3e7n]]Crystallographic visualisation of these two proteins has revealed that the biological unit of each is a homo-decameric oligomer, which has a ring--like quaternary structure. The complete oligomer of 3e7n is shown in figure 4. PQS analysis of both structures suggests that this decameric assembly is correct for each (EMBL-EBi PQS 2008). | |||
Note the ring-like structure of the oligomer, which contains distinct upper and lower pentameric sub-rings which are rotated slightly relative to each other, such that no more than three subunits coincide at any one junction. | |||
===Alignment visualisation=== | |||
[[Image:2ob5_triple_align.jpg|left|thumbnail|Figure 5. Structural alignment of 2ob5 (red/blue), 1ogd (green) and 3e7n (yellow-green)]][[Image:multiseq_align.jpg|right|thumbnail|Figure 19. Multiseq alignment of 2ob5, 1ogD and 3e7n, coloured by BLOSUM60 of residue conservation. Blue residues are highly conserved, red are divergent.]] | |||
Structural similarity between the monomers of both proteins and 2ob5A is high, despite sequence identity values of less than 20%. To confirm the high structural identity of the Dali results (z=17.9 and 16.9 for 3e7n-c and 1ogd-D, respectively), the proteins were aligned using MultiSeq for VMD and overlaid (Roberts et al 2006, Humphrey et al 1996)) (Figure 19.). From visual analysis, the strength of the alignment is clear. | |||
In Figure 5, 2ob5 is coloured by a BLOSUM60 plot of residue conservation. The prevalence of blue residues in the internal beta-sheets indicates high sequence conservation in those regions. This is supported by the molecule's RbsD-like fold classification, as the 6-strand beta-sheet structure is highly conserved in RbsD proteins. | |||
==Ligand binding site== | |||
As a result of this considerable structural similarity, we conducted an in depth comparison of 2ob5A to the better characterised 3e7n and 1ogd. | |||
===1ogD and 3e7n=== | |||
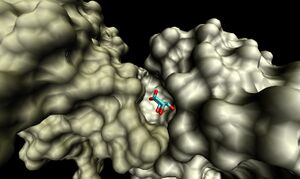
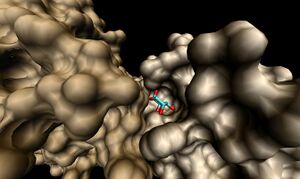
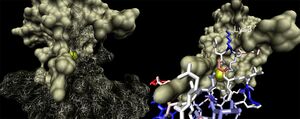
[[Image:1ogD_lig_surf.jpg|right|thumbnail|Figure 6. Surface representation of the 1ogD ribose-binding cleft ]] | |||
The binding sites of 1ogd are known, and the protein has been crystallised bound to its ribose-D ligand in a pentameric ring quaternary structure (Kim et al. 2003). The oligomerisation of the protein appears necessary for the binding of this ligand, as the active site lies in a cleft between proteins adjacent on the same ring (Figure 6.). | |||
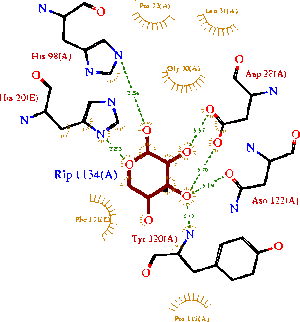
[[Image:1ogd_ligplot.gif|left|thumbnail| Figure 7. LIGPLOT of 1ogD ribose-binding residues]] A LIGPLOT analysis of the 1ogd ribose-D binding site is shown in Figure 7 (Wallace et al. 1995). | |||
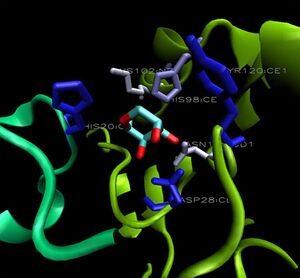
[[Image:1ogd_key-lig.jpg|right|thumbnail|Figure 8. 3D arrangement of 1ogD ribose-binding residues, coloured by sequence conservation]] | |||
His20 is donated by the adjacent monomer. The residue arrangement is shown in 3D in at right. | |||
Residues are again coloured by sequence conservation. All five residues are conserved in 3e7n, further supporting their involvement in binding of ribose-D. | |||
===Target protein=== | |||
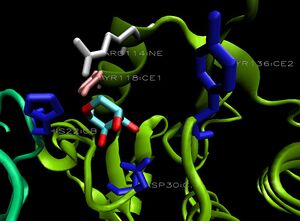
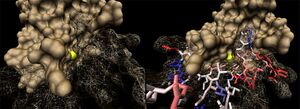
[[Image:2ob5_key-lig.jpg|left|thumbnail| Figure 9. Arrangement of 2ob5 ribose-binding residues, coloured by sequence conservation]] | |||
2ob5A was not crystallised with ligand present. As such, it is uncertain whether it too is a ribose binding protein. His20, Tyr120 and Asp28 on 1ogd are all conserved in 2ob5A. Asn122 appears to have undergone a deletion judging from the sequence alignment, and His98 and Lys102 have been substituted for by Arg and Tyr residues, respectively. These differences may suggest a slightly different specificity. However, the 3D Multiseq alignment of 2ob5 with 1ogD and 3e7n retained the binding amino acids in position. Superimposition of the 1ogD ligand onto an aligned 2ob5A (Figure 9.) suggests that the binding mode and/or ligand of 2ob5 is similar. | |||
[[Image:2ob5_lig_surf.jpg|right|thumbnail| Figure 10. Surface representation of potential 2ob5 ligand-binding cleft, with ribose-D]] | |||
Visualisation of the corresponding surfaces indicate that a binding cleft is still present on 2ob5A (Figure 10.). | |||
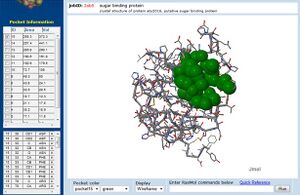
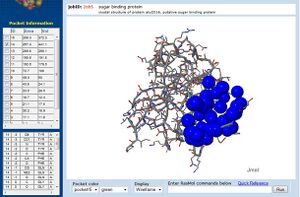
[[Image:CASTp_2ob5_lig.jpg|left|thumbnail| Figure 11. CASTp representation of putative binding pockets in 2ob5]] | |||
Additionally, CASTp analysis predicts the monomer's highest surface-area cleft at this site (Dundas et al. 2006) (figure 11.). A large pocket is also identified by CASTp in 1ogd, although this protein's pentameric crystallisation allows its predicted cleft to include the adjacent His20 residue. | |||
==Ion-coordination site== | |||
===Key residues=== | |||
[[Image:1ogd_clplot.gif|right|thumbnail| Figure 12. LIGPLOT of 1ogD ion coordination]] | |||
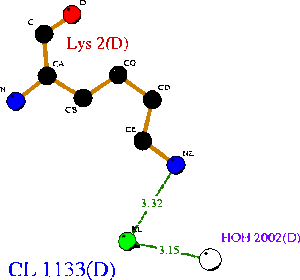
1ogd was cocrystallised with an ion, tentatively labelled as a chloride in this case. The ion coordinating site lies at the amino-terminus of the protein. Ligplot analysis gives Lys2 as a key residue for ion coordination (Wallace et al. 1995) (Figure 12.). | |||
[[Image:1ogd_dec_rib.jpg|left|thumbnail| Figure 13. Ribbon visualisation of simulated 1ogD decamer, showing ligands]] | |||
Figure 13. shows the 3D alignment of two 1ogD pentamers onto the 3e7n decameric scaffold. The chloride ion is visible in the centre of the facing side. Bound Ribose-D molecules are also seen. | |||
[[Image:1ogd_cl_cage.jpg|right|thumbnail|Figure 14. 1ogD ion coordination site, coloured by sequence conservation. Coordinating lysine residues are highlighted]] | |||
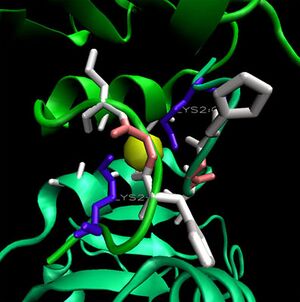
Ions appear coordinated by two adjacent monomers; specifically by the residue Lys 2. Unlike the ribose binding site, however, the ion binding cleft is formed by subunits on different pentameric rings; one from the upper and one lower (Kim et al. 2003)(clear in Figure 13.). LPC analysis indicates that the Lys2 residues combine with hydrophobic residues to coordinate the ion (Sobolev et al. 1999). The resulting structure appears to form an ion cage (Kim et al. 2003) (Figure 14). | |||
Although the individual hydrophobic residues are poorly conserved, the cage structure and coordinating lysines are also present on 3e7n. It is possible that this ion contributes to the stability of the decameric ring, existing as it does at the interfaces between top and bottom halves of the oligomer. | |||
[[Image:2ob5_cl_cage.jpg|left|thumbnail|Figure 15. Putative 2ob5 ion-coordination site. Key reidues are highlighted.]] | |||
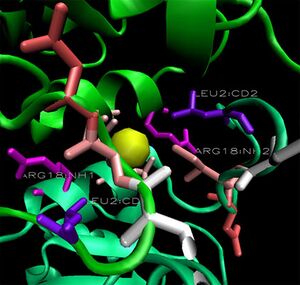
When monomers of 2ob5 are superimposed on the ring structure of 1ogd or 3e7n, the target protein appears to retain its ion cage structure (Figure 15). However, the critical coordinating lysine is not present. Lys2 has been replaced with a hydrophobic leucine, which cannot coordinate a Cl ion. An additional positive residue, arginine - replacing Ala16 on 1ogd - is present, but appears in the wrong orientation. It is possible that 2ob5A does not have the ability to coordinate an ion at this site. | |||
===Surrounding surfaces=== | |||
[[Image:2ob5-1ogd_cl_mesh.jpg|left|thumbnail| Figure 16. Surface representations of 1ogDion coordination site. Residues coloured by conservation.]] | |||
Surface representations of the 1ogD Cl-binding cleft indicate a buried coordination site (Figure 16.). This corresponds to the predicted ion cage structure. The interface between the two subunits covers a comparatively large surface area at this site, and the region as a whole is comparatively well conserved. Of particular note is the residue Lys3 (highlighted on Figure 16.), which is conserved across all three crystallised proteins and lies in an extended cleft in the adjacent monomer. | |||
[[Image:2ob5_cl_mesh.jpg|right|thumbnail|Figure 17. Surfaces of the 2ob5 ion coordination site. 2ob5 is shown here with the Cl ion present. As discussed, this may not be the case in situ.]] | |||
This residue and its cleft are conserved in 2ob5 (Figure 17.), although individual sequence conservation at the cleft is not high. | |||
This, along with a number of corresponding cleft-ligand-like features at the subunit interface of 2ob5, suggests that 2ob5 exists in the same decameric conformation as 1ogd and 3e7n despite the potential absence of the ion. | |||
[[Image:CASTp_2ob5_cl.jpg|left|thumbnail| Figure 18. CASTp result showing potential ion-binding region]] | |||
CASTp analyses supports this (Figure 18.), as the molecule's second-largest predicted binding surface lies around the putative ion-coordination site (Dundas et al. 2006). | |||
==Sequence conservation== | |||
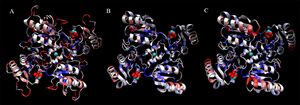
[[Image:2ob5_triple-set.jpg|right|thumbnail|Figure 20. Ribbon diagram showing similarities between 2ob5 (A), 1ogD (B) and 3e7n (C), coloured by BLOSUM60 of sequence conservation and shown with attached ligands]] | |||
Finally, shown in Figure 20. are representations of a four-subunit face of each protein, demonstrating potential binding sites for D-ribose and Cl. Residues are coloured by sequence conservation between these three proteins. | |||
It is again clear that the structural alignment is strong, even at the quaternary level. Additionally, it can be seen that although 2ob5 has many areas of low conservation, these generally correspond with variable loop regions, and that the key secondary-structural elements remain remarkably constant. | |||
=='''Function'''== | |||
===Blast Search=== | |||
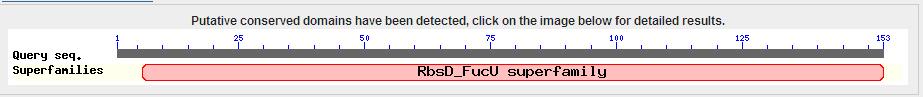
[[Image:blasthp.jpg]] | |||
The results from the blast search showed a conserved FucU/RbsD domain. | |||
===String Analysis=== | |||
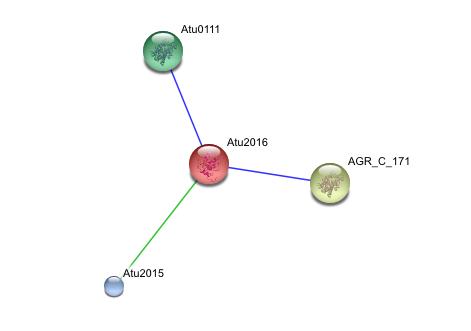
[[Image:String.jpg]] | |||
This string analysis shows possible protein - protein interactions. The red protein in the middle represents the target protein and the three surrounding proteins represent interacting proteins. | |||
The larger yellow and green proteins may be releated due to cooccurrence, where as the smaller blue protien may be related due to repeatedly occurring in close proximity to the target protein, however the posible interactions shown in the analysis are inconclusive and unsubstantiated. experimental evidence is needed to prove such relationships<br> | |||
===Interpro Scan result=== | |||
[[Image:Interpro1.gif]] | |||
This image shows the results of a InterProScan. The InterProScan search identifies sequence motifs from several databases.These results show that the target protein belongs to the RbsD/FucU superfamily (Purple and red lines) | |||
The gray line shows an possible binding histadine site. | |||
===PDB Sequence Match=== | |||
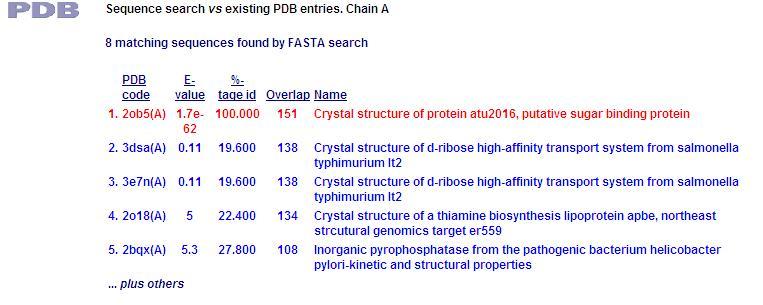
[[Image:PDB1.jpg]] | |||
This PDB scan compares structure alignment. The highest structural match with our sequence is shown to be involved in an d-ribose high affinity transport system. This supports the theory that our hypothetical protein is involved in the ABC transport system of ribose in prokaryote cells. | |||
[[Hypothetical protein Abstract | Abstract ]] | [[Hypothetical protein Introduction | Introduction]] | [[Hypothetical protein Method| Method]] | | |||
[[Hypothetical protein Results | Results]] | [[Hypothetical protein Discussion | Discussion]] | [[Hypothetical protein Conclusion | Conclusion ]] | [[Hypothetical protein References | References ]]<BR> | |||
[[hypothetical protein LOC282969 isoform 1 | Go Back To Main Page]] | [[hypothetical protein LOC282969 isoform 1 | Go Back To Main Page]] | ||
Latest revision as of 01:54, 16 June 2009
Evolution
Sequences
Sequences (FASTA format) gathered for MSA (including query), available via the following link: [1]
Multiple Sequence Alignment
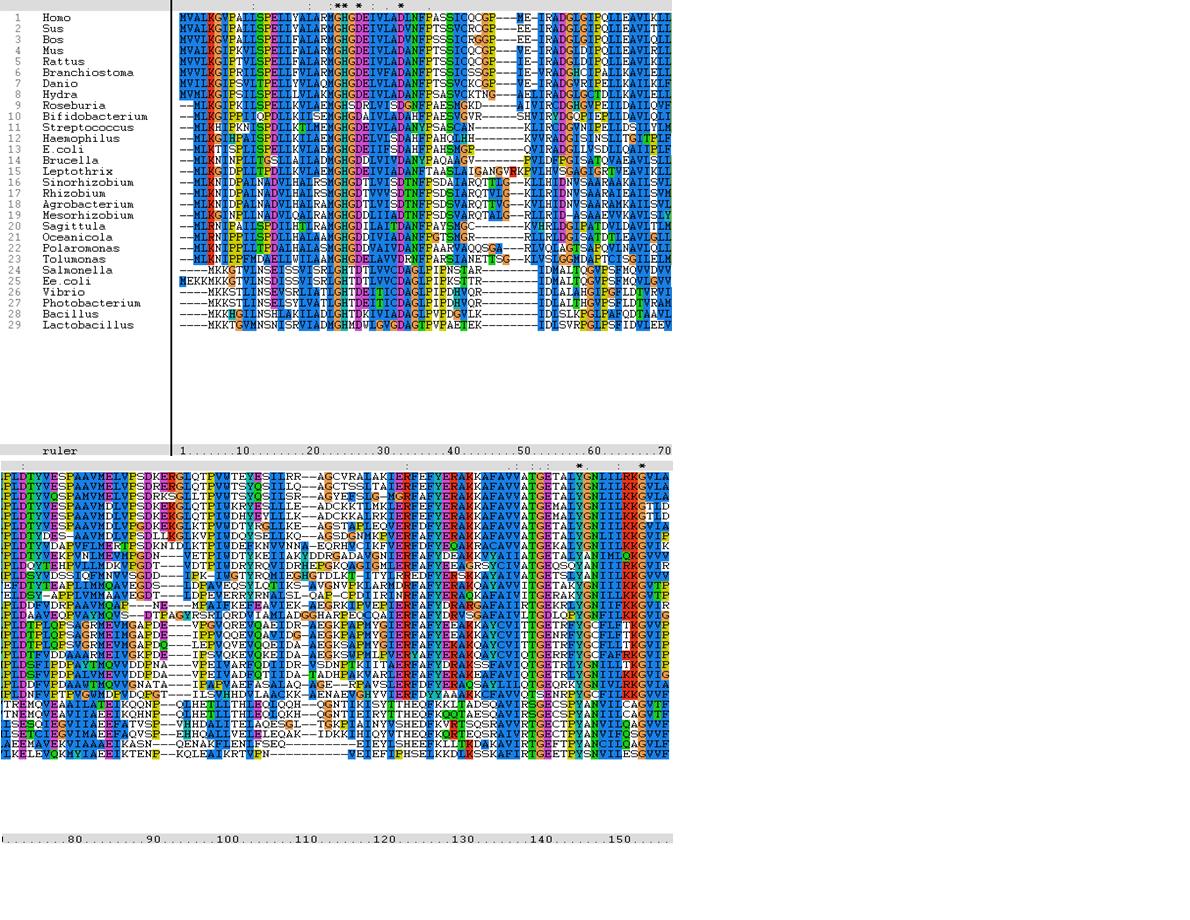 Multiple sequence alignment generated using Clustalx 1.83 [1].
Multiple sequence alignment generated using Clustalx 1.83 [1].
Original Curved Bootstrapped Tree
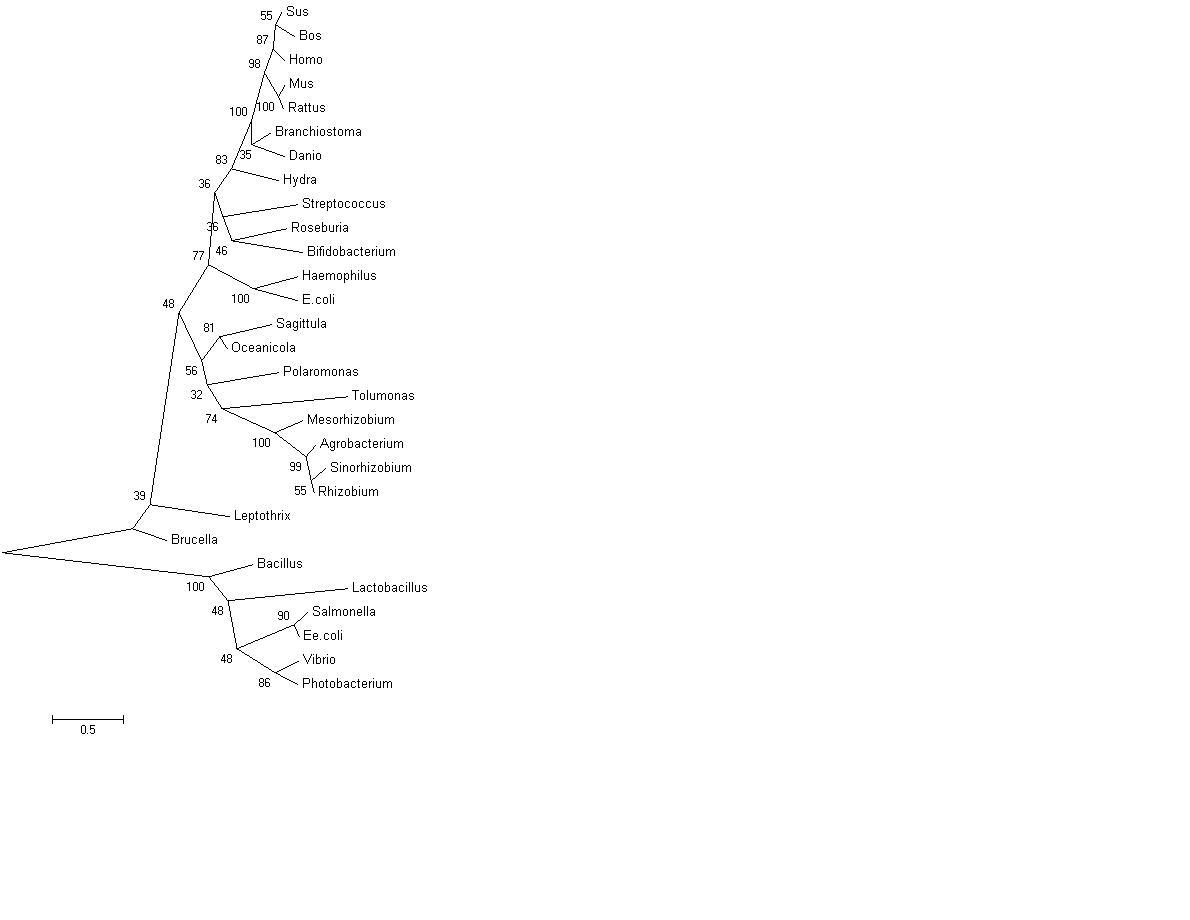 Curved phylogenetic tree with bootstrap values, obtained using MEGA 4.0 [2]
Curved phylogenetic tree with bootstrap values, obtained using MEGA 4.0 [2]
Radiation Tree showing relevant bootstrap values
Phylogenetic tree representing radiation, obtained using MEGA 4.0 [2] and manually edited in Microsoft Power Point 2007.
Structure
Crystal structure
Initial structural analyses indicate that 2ob5A is a member of the RbsD/FucU protein superfamily (Sanger Institute 2001), and is of the RbsD-like fold classification (Murzin et al. 1995). It is a globular protein composed of five alpha-helixes arranged around six internal beta strands in the tri-layered a/b/a fold characteristic of RbsD-like proteins (EMBL-EBI PDBSum 2007) (Figure 2).
Structural alignment searches
Dali search
Early structural alignments (Holm & Sander 1993) supported this classification, returning ribose-transport proteins as high similarity hits. Of the structures found, the majority of high-significance (z > 15) matches were variations on two prokaryotic RbsD proteins, 3e7n (D-ribose high affinity transport system, S. Typhi) and 1ogD (high affinity ribose transporting protein, B. Subtillis). These two crystal structures are shown in figure 3 (RCSB 2008).
Structures of matching proteins
Crystallographic visualisation of these two proteins has revealed that the biological unit of each is a homo-decameric oligomer, which has a ring--like quaternary structure. The complete oligomer of 3e7n is shown in figure 4. PQS analysis of both structures suggests that this decameric assembly is correct for each (EMBL-EBi PQS 2008).
Note the ring-like structure of the oligomer, which contains distinct upper and lower pentameric sub-rings which are rotated slightly relative to each other, such that no more than three subunits coincide at any one junction.
Alignment visualisation
Structural similarity between the monomers of both proteins and 2ob5A is high, despite sequence identity values of less than 20%. To confirm the high structural identity of the Dali results (z=17.9 and 16.9 for 3e7n-c and 1ogd-D, respectively), the proteins were aligned using MultiSeq for VMD and overlaid (Roberts et al 2006, Humphrey et al 1996)) (Figure 19.). From visual analysis, the strength of the alignment is clear.
In Figure 5, 2ob5 is coloured by a BLOSUM60 plot of residue conservation. The prevalence of blue residues in the internal beta-sheets indicates high sequence conservation in those regions. This is supported by the molecule's RbsD-like fold classification, as the 6-strand beta-sheet structure is highly conserved in RbsD proteins.
Ligand binding site
As a result of this considerable structural similarity, we conducted an in depth comparison of 2ob5A to the better characterised 3e7n and 1ogd.
1ogD and 3e7n
The binding sites of 1ogd are known, and the protein has been crystallised bound to its ribose-D ligand in a pentameric ring quaternary structure (Kim et al. 2003). The oligomerisation of the protein appears necessary for the binding of this ligand, as the active site lies in a cleft between proteins adjacent on the same ring (Figure 6.).
A LIGPLOT analysis of the 1ogd ribose-D binding site is shown in Figure 7 (Wallace et al. 1995).
His20 is donated by the adjacent monomer. The residue arrangement is shown in 3D in at right. Residues are again coloured by sequence conservation. All five residues are conserved in 3e7n, further supporting their involvement in binding of ribose-D.
Target protein
2ob5A was not crystallised with ligand present. As such, it is uncertain whether it too is a ribose binding protein. His20, Tyr120 and Asp28 on 1ogd are all conserved in 2ob5A. Asn122 appears to have undergone a deletion judging from the sequence alignment, and His98 and Lys102 have been substituted for by Arg and Tyr residues, respectively. These differences may suggest a slightly different specificity. However, the 3D Multiseq alignment of 2ob5 with 1ogD and 3e7n retained the binding amino acids in position. Superimposition of the 1ogD ligand onto an aligned 2ob5A (Figure 9.) suggests that the binding mode and/or ligand of 2ob5 is similar.
Visualisation of the corresponding surfaces indicate that a binding cleft is still present on 2ob5A (Figure 10.).
Additionally, CASTp analysis predicts the monomer's highest surface-area cleft at this site (Dundas et al. 2006) (figure 11.). A large pocket is also identified by CASTp in 1ogd, although this protein's pentameric crystallisation allows its predicted cleft to include the adjacent His20 residue.
Ion-coordination site
Key residues
1ogd was cocrystallised with an ion, tentatively labelled as a chloride in this case. The ion coordinating site lies at the amino-terminus of the protein. Ligplot analysis gives Lys2 as a key residue for ion coordination (Wallace et al. 1995) (Figure 12.).
Figure 13. shows the 3D alignment of two 1ogD pentamers onto the 3e7n decameric scaffold. The chloride ion is visible in the centre of the facing side. Bound Ribose-D molecules are also seen.
Ions appear coordinated by two adjacent monomers; specifically by the residue Lys 2. Unlike the ribose binding site, however, the ion binding cleft is formed by subunits on different pentameric rings; one from the upper and one lower (Kim et al. 2003)(clear in Figure 13.). LPC analysis indicates that the Lys2 residues combine with hydrophobic residues to coordinate the ion (Sobolev et al. 1999). The resulting structure appears to form an ion cage (Kim et al. 2003) (Figure 14).
Although the individual hydrophobic residues are poorly conserved, the cage structure and coordinating lysines are also present on 3e7n. It is possible that this ion contributes to the stability of the decameric ring, existing as it does at the interfaces between top and bottom halves of the oligomer.
When monomers of 2ob5 are superimposed on the ring structure of 1ogd or 3e7n, the target protein appears to retain its ion cage structure (Figure 15). However, the critical coordinating lysine is not present. Lys2 has been replaced with a hydrophobic leucine, which cannot coordinate a Cl ion. An additional positive residue, arginine - replacing Ala16 on 1ogd - is present, but appears in the wrong orientation. It is possible that 2ob5A does not have the ability to coordinate an ion at this site.
Surrounding surfaces
Surface representations of the 1ogD Cl-binding cleft indicate a buried coordination site (Figure 16.). This corresponds to the predicted ion cage structure. The interface between the two subunits covers a comparatively large surface area at this site, and the region as a whole is comparatively well conserved. Of particular note is the residue Lys3 (highlighted on Figure 16.), which is conserved across all three crystallised proteins and lies in an extended cleft in the adjacent monomer.
This residue and its cleft are conserved in 2ob5 (Figure 17.), although individual sequence conservation at the cleft is not high. This, along with a number of corresponding cleft-ligand-like features at the subunit interface of 2ob5, suggests that 2ob5 exists in the same decameric conformation as 1ogd and 3e7n despite the potential absence of the ion.
CASTp analyses supports this (Figure 18.), as the molecule's second-largest predicted binding surface lies around the putative ion-coordination site (Dundas et al. 2006).
Sequence conservation
Finally, shown in Figure 20. are representations of a four-subunit face of each protein, demonstrating potential binding sites for D-ribose and Cl. Residues are coloured by sequence conservation between these three proteins. It is again clear that the structural alignment is strong, even at the quaternary level. Additionally, it can be seen that although 2ob5 has many areas of low conservation, these generally correspond with variable loop regions, and that the key secondary-structural elements remain remarkably constant.
Function
Blast Search
The results from the blast search showed a conserved FucU/RbsD domain.
String Analysis
This string analysis shows possible protein - protein interactions. The red protein in the middle represents the target protein and the three surrounding proteins represent interacting proteins.
The larger yellow and green proteins may be releated due to cooccurrence, where as the smaller blue protien may be related due to repeatedly occurring in close proximity to the target protein, however the posible interactions shown in the analysis are inconclusive and unsubstantiated. experimental evidence is needed to prove such relationships
Interpro Scan result
This image shows the results of a InterProScan. The InterProScan search identifies sequence motifs from several databases.These results show that the target protein belongs to the RbsD/FucU superfamily (Purple and red lines) The gray line shows an possible binding histadine site.
PDB Sequence Match
This PDB scan compares structure alignment. The highest structural match with our sequence is shown to be involved in an d-ribose high affinity transport system. This supports the theory that our hypothetical protein is involved in the ABC transport system of ribose in prokaryote cells.
Abstract | Introduction | Method |
Results | Discussion | Conclusion | References