LOC56985 results: Difference between revisions
No edit summary |
No edit summary |
||
| Line 4: | Line 4: | ||
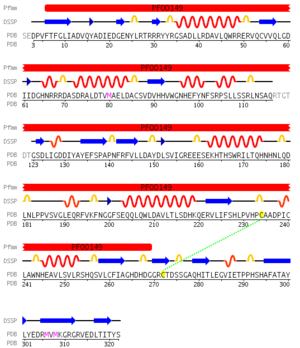
Pfam analysis shows a common domain to all these proteins as being a calcineurin-like phosphoesterase (PF00149). SCOP classification (Murzin et al 1995) of the protein shows it to be an Alpha and Beta (a + b) protein with a 4-layer sandwich fold of alpha/beta/beta/alpha, in the family of puple acid phosphatases (56301). The secondary structure assignment (Kabsch & Sander, 1983) show that the protein contains 20 beta-sheets, 12 alpha-helices and a single disulfide bond between Cys272 and Cys234 (Figure 2); the Pfam domain is shown to tretch from Phe6 to Gly269. | Pfam analysis shows a common domain to all these proteins as being a calcineurin-like phosphoesterase (PF00149). SCOP classification (Murzin et al 1995) of the protein shows it to be an Alpha and Beta (a + b) protein with a 4-layer sandwich fold of alpha/beta/beta/alpha, in the family of puple acid phosphatases (56301). The secondary structure assignment (Kabsch & Sander, 1983) show that the protein contains 20 beta-sheets, 12 alpha-helices and a single disulfide bond between Cys272 and Cys234 (Figure 2); the Pfam domain is shown to tretch from Phe6 to Gly269. | ||
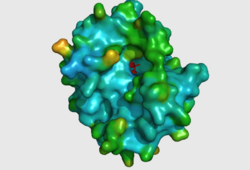
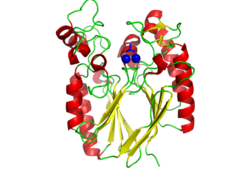
The crystal structure of 2nxf shows that it binds 4Zn, PO4 and an ethanol molecule. The PO4 binds in the largest cleft in the protein at an active site that contains two Zn atoms. This site shows three identical residues across the top five protein matches by Dali. This includes the human homolog as well as the Enterbacter aerogenes protein. The sequence of residues is GNH, with a highly conserved acidic amino acid adjacent and down stream of this pattern. This same sequences were placed into the scorecons server to analyse how informative the alignment was; it returned a result of 99.2%, which rates this alignment as very diverse and informative. CASTp predicts that the protein contains a number of cavities the largest of which has a volume of 2043.1 covering an area of 950.8. This cavity is very large compared to the others predicted by CASTp. PyMOL analysis of this pocket shows that it is the binding site for the PO4 ligand and contains two Zn atoms buried in the pocket. | The crystal structure of 2nxf shows that it binds 4Zn, PO4 and an ethanol molecule. The PO4 binds in the largest cleft in the protein at an active site that contains two Zn atoms. This site shows three identical residues across the top five protein matches by Dali. This includes the human homolog as well as the Enterbacter aerogenes protein. The sequence of residues is GNH, with a highly conserved acidic amino acid adjacent and down stream of this pattern. This same sequences were placed into the scorecons server to analyse how informative the alignment was; it returned a result of 99.2%, which rates this alignment as very diverse and informative. CASTp predicts that the protein contains a number of cavities the largest of which has a volume of 2043.1 covering an area of 950.8. This cavity is very large compared to the others predicted by CASTp. PyMOL analysis of this pocket shows that it is the binding site for the PO4 ligand and contains two Zn atoms buried in the pocket. [[Image:Surface view of ligand binding site.png|left|thumb|250px|Figure 3: The surface view of the protein showing the metal binding site in a large cavity on the protein.This cavity is bridged so that it appears to be a donut shape, this formation is not picked up in the ribbon view of 2NXF which shows a clear gap in the secondary structure of the protein. Many other smaller cavities can also be seen in the protein. Two Zinc atoms are shown as red spheres interacting with the phosphate ion]][[Image:2NXF ribbon structure2.png|centre|thumb|250px|Figure 4:Ribbon view of 2NXF showing the 4-layer alpha/beta sandwich structure described by the SCOP classification. The structure shows that the outer layers of the protein are dominated by alpha helices (red coils), while the center of the protein consists of two distinct layers of beta sheets (yellow arrows).]] | ||
[[LOC56985_abstract| Abstract]] | [[LOC56985_intro| Introduction]] | [[LOC56985_results| Results]] | [[LOC56985_discussion| Discussion]] | | [[LOC56985_abstract| Abstract]] | [[LOC56985_intro| Introduction]] | [[LOC56985_results| Results]] | [[LOC56985_discussion| Discussion]] | | ||
Revision as of 01:47, 31 May 2008
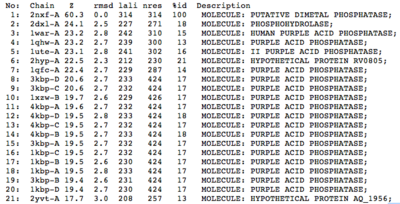
A structural comparison search of the Danio rerio ortholog (2nxf) showed that the gene product had its greatest similarity to a phosphorhydrolase and to various purple acid phosphatases (figure 1). All of these protein matches showed little sequence similarity to the 2nxf however the Z-score of these proteins were significant based on the guide given on the Dali server that Z-scores below 2.5 are insignificant matches. The top hit, 2dxl-A, showed a Z-score of 24.1; other members in the top five matches again showed Z-scores of above 20. Dali works by fitting the two structures together and determining how far apart each of the atoms are from one another to give a root mean square deviation (rmsd), even in the lowest of the top 20 matches the rmsd is 3.0 or below.
Pfam analysis shows a common domain to all these proteins as being a calcineurin-like phosphoesterase (PF00149). SCOP classification (Murzin et al 1995) of the protein shows it to be an Alpha and Beta (a + b) protein with a 4-layer sandwich fold of alpha/beta/beta/alpha, in the family of puple acid phosphatases (56301). The secondary structure assignment (Kabsch & Sander, 1983) show that the protein contains 20 beta-sheets, 12 alpha-helices and a single disulfide bond between Cys272 and Cys234 (Figure 2); the Pfam domain is shown to tretch from Phe6 to Gly269.
The crystal structure of 2nxf shows that it binds 4Zn, PO4 and an ethanol molecule. The PO4 binds in the largest cleft in the protein at an active site that contains two Zn atoms. This site shows three identical residues across the top five protein matches by Dali. This includes the human homolog as well as the Enterbacter aerogenes protein. The sequence of residues is GNH, with a highly conserved acidic amino acid adjacent and down stream of this pattern. This same sequences were placed into the scorecons server to analyse how informative the alignment was; it returned a result of 99.2%, which rates this alignment as very diverse and informative. CASTp predicts that the protein contains a number of cavities the largest of which has a volume of 2043.1 covering an area of 950.8. This cavity is very large compared to the others predicted by CASTp. PyMOL analysis of this pocket shows that it is the binding site for the PO4 ligand and contains two Zn atoms buried in the pocket.
Abstract | Introduction | Results | Discussion | Conclusion | Method | References