N-myc downstream-regulated gene 2 isoform b
Introduction
The N-myc down regulated gene (NDRG) family belonging to the alpha/beta hydrolase superfamily consists of four members NDRG 1,2 ,3 and 4 where they share 50-60% homology. NDRG is highly conserved in a variety of eukaryotes suggesting functions in cell differentiation and proliferation. Studies have shown that NDRG2 gene is down regulated by Myc proteins via transcriptional repression therefore, high levels of NDRG2 was observed as Myc expression was reduced in differentiated cells. Myc encodes a transcription factor that binds to DNA of other genes regulating cellular differentiation. Mutations in MYC results in failure of protein to bind correctly to DNA resulting in uncontrolled cell division (cancer).Interestingly, studies have shown that NDRG2 is not repressed by N-myc unlike NDRG1. However, the mechanism in which NDRG2 is not suppressed is still unknown. NDRG2 is a hypoxia responsive gene which induces the apoptosis of tumor cell. To further investigate the role and function of NDRG2 the structure, function and evolution of NDRG2 was analyzed and compared with proteins with similar homology and structure that have been discovered using a range of bioinformatics programs and databases available online.
Methods
Structural analysis of NDRG 2 protein.
For structure analysis PyMol was used to view PDB structure (2qmq) of N-myc downstream regulated 2. Dali was then used for structural comparison of our protein with other proteins with similar or closely related structure. A secondary structure matching program at EMBL-EBI(http://www.ebi.ac.uk/msd-srv/ssm/cgi-bin/ssmserver) was used to examine our protein from PDB to find similarities within whole SCOP (Structural Classification Of Proteins) archive. Chloroperoxidase protein structure was found and used as a comparison due to its significant Z-score and low Rmsd value. PyMol was then used to structurally align both proteins together comparing and noting differences in structure.
Determining Function of Protein
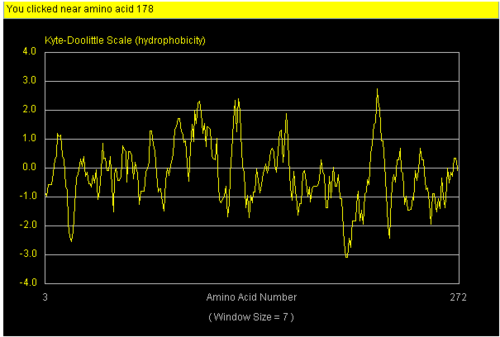
The NCBI site (http://www.ncbi.nlm.nih.gov/) was used to perform literature search to try and discover the function of NDRG-2. Profunc tool(www.ebi.ac.uk/thornton-srv/databases/ProFunc/) was use to determine the most likely biochemical function of our protein based on its 3 dimensional structure. Strings database was then used to correlate its interaction with other proteins. Hydropathy plot and hydrophobicity calculator was used to mark specific hydrophobic and hydrophilic region on surface of our protein.
Determining Evolutionary relationship.
PSI-BLAST search was performed using the NCBI web interface (http://www.ncbi.nlm.nih.gov/blast) against the non-redundant database to search for homologous proteins of Human NDRG-2. Amino acid sequences from resulting homologous proteins were saved as FASTA format. The amino acid sequences were then aligned using ClustalX. The alignment was exported as a phylip file extension and subsequently used in creating a phylogenetic tree using Phylip software. The phylogentic tree was then imported into Figtree and edited to highlight different kingdoms of organisms.
Results
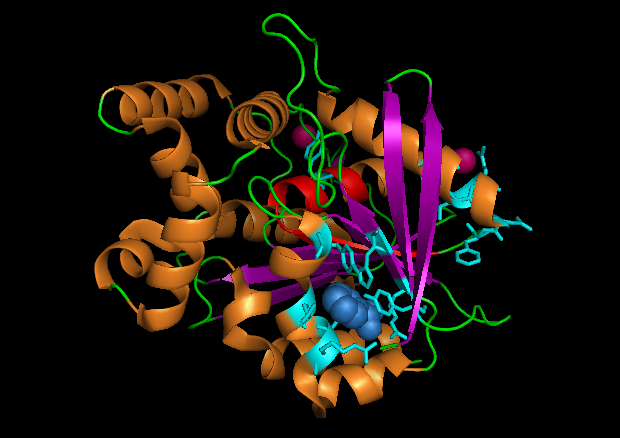
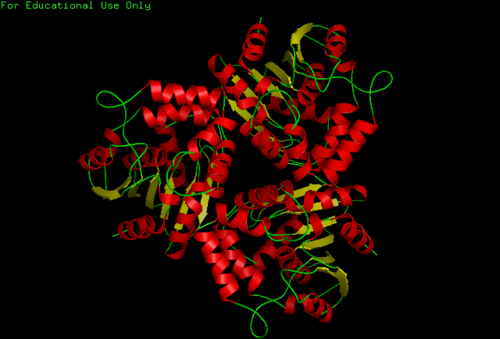
The NDRG2 molecule was related to the a/b hydrolase superfamily with a key characteristic where the second B-sheet of the molecule is anti-parallel compared to the rest of the B-sheet. This proteins consists mainly of alpha 15 helices and 9 beta sheets
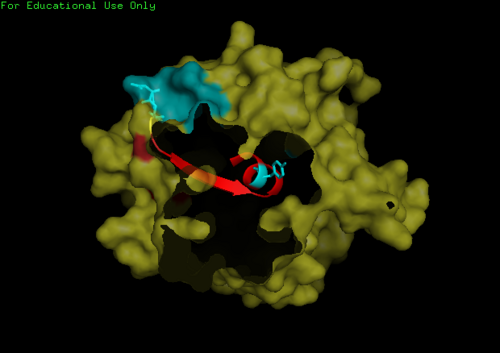
This highly conserved region is located at the core of the NDRG2 protein linked to ligands directly to the surface of the protein.
We found a region with hydrophobic characteristic situated at the surface of the protein (amino acid residue 101-178) which plays a crucial role in its translocation from the cytoplasm into the nucleus where the hydrophobic region binds to nuclear membrane which facilitates its diffusion into the nucleus (Fig 3,4).
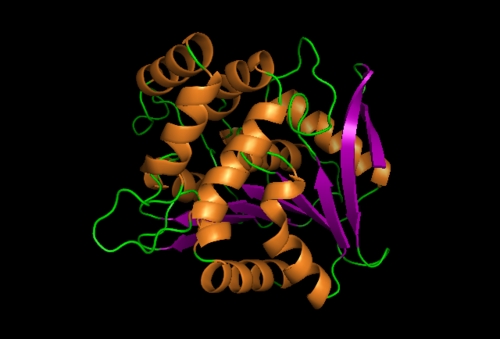
A single Chloroperoxidase domain was found to be most closely related to NDRG2 structure where each of its domains best matches overall structure of our protein based on RMSD (Root Mean Square Deviation) values.
The NDRG-2 gene is down regulated by MYC (myelocytomatosis) via transcriptional repression. However if MYC is mutated then incorrect binding to other genes is observed which results in incorrect cell differentiation of gene products. This can lead to the growth of tumor cells.
PSI-BLAST results showed a mixture of predicted, hypothetical and proven proteins. The majority of proteins had extremely high identity NDRG-2. The Bit scores and E-values also showed similar results. 42 proteins were selected to represent a wide range of species from every kingdom. Overall the selected proteins showed high identity to Human NDRG-2. A few exceptions exist like for example Streptomyces Lividans protein which showed relatively low identity but still high similarity and was included because structurally the protein is extremely similar to human NDRG-2. PSI-BLAST results can be found here.
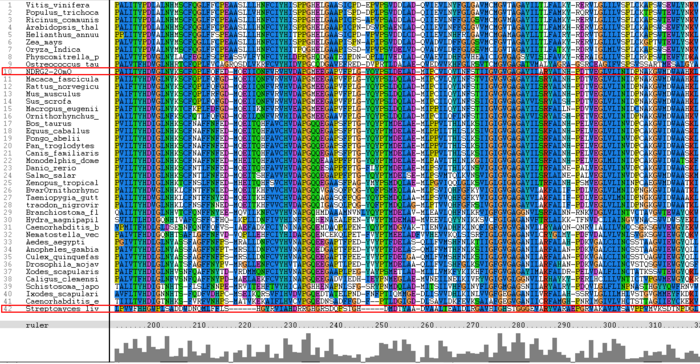
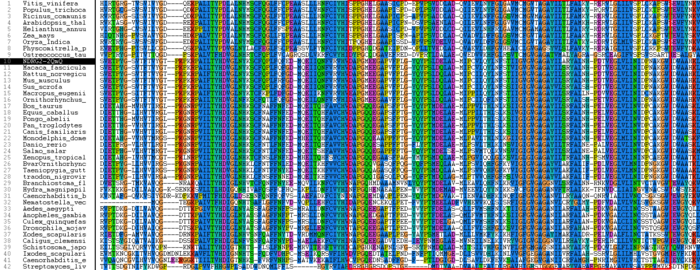
The multiple sequence alignment (MSA) computed using ClustalX shown in Figure 9 shows high identity between all animals and plants however insects, parasites and bacteria show little to average identity but high similarity. Highlighted in Figure 9 is the human NDRG-2 protein, which has extremely high identity with other mammals. Also highlighted is an enzyme (chloroperoxidase) from [i]Streptomyces Lividans[/i], which as stated above contains low identity but is structurally very similar to human NDRG-2. Several highly conserved regions can be observed throughout the MSA, of interest is the translocation region which is highlighted in Figure 10. The translocation region is 77 amino acid residues long and is highly conserved throughout almost all of the organisms.
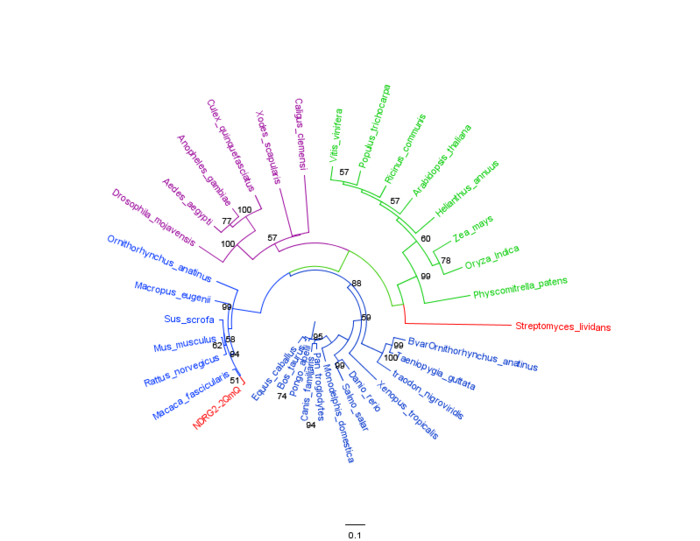
The phylogenic tree shown in Figure 11 shows 3 distinct branches. The green branch consists predominately of the plants however [i]Streptomyces Lividans[/i] is part of this branch and is coloured red. The blue branch is composed completely of animals and birds, it is subsequently the largest of the 3 branches. The blue branch also contains the human NDRG-2 gene which is coloured in red. The third branch which is coloured purple completely comprises of insects. The phylogenetic tree indicates conservation of the NDRG-2 gene throughout the animal kingdom as can be seen by the high bootstrap values. There is also conservation of NDRG-2 in plants and insects, however it should be noted that [i]Streptomyces Lividans[/i] is part of the plant branch which is highly unusual. From examination of the branch positions it can be determined that the insects (purple branch) seem to be more closely related to plants (green branch) than animals and birds (blue branch). It is evident that [i]Streptomyces Lividans[/i] has low sequence similarity to plants as the branch (coloured red) is diverging away.
Discussion
Function of Chloroperoxidase is to hydrolyze hydrogen peroxide as high concentrations of it have negatives effects in cells. E.g. accumulation of H2O2 In plants cells have damaging effects DNA, lipid membranes and organelles and plants rely on these enzymes to prevent accumulation of H2O2. However, presence of H2O2 also plays a role in plant defenses against pathogens. Further evidence suggests that NDRG 2 is believed to play a role in the regulation of H2O2 levels based on the overlay of human NDRG-2 protein with chlorrperoxidase from Streptomyces Lividans. This is due to low RMSD values indicating the best superimposition between both 3-d structures whihc in turn suggests that the homology of both structure may be correlated to its similarity in function.
The presences of many hydrophobic residues on the surface of NDRG-2 shows that it is needed to facilitate entry pass the nuclear membrane into the nucleus. The hydrophobic region binds to hydrophobic nuclear membrane regions and via homophilic binding facilitates its endocytosis into the nucleus.Within this region consists of ligands which connects directly to the conserved region core of the protein which may also play a role in faculitating its translocation into the nucleus where this could server as a recognition signal for its translocation into the nucleus [1].
Based on structure of chloroperoxidase NDRG-2 protein could be active as a trimer suggesting that multiple domains is required to form a full functional molecule where the active site is formed via interaction of multiple domains.e.g formation of a catalytic triad found in protease enzyme which functions in the hydrolysis of peptide bonds due to specific amino acid residues located in the active site [14].No functions were found for organic residues (Benzoic acid and Nonaethylene glycol) present on proteins structure. Pressence of these organic residues could be due to remaining residues following crystallization of structure as no such residues were present on molecules with similar alpha/beta hydrolase molecules.
The NDRG2 function is to down-regulate the mutated myc gene to inhibit the tumor cell proliferation. NDRG2 is a hypoxia induced gene that functions in apoptosis to kill the tumor cells, in the presence of lower level of oxygen, NDRG2 is more expressed. Hypoxia also induced the translocation of NDRG2 from cytoplasm to nucleus of tumor cells, although the nuclear localisation signal (NLS) as a nuclear import element is not identified in NDRG2 protein, but it’s speculated that NDRG2 protein has its own motif for the nuclear translocation. [1] Lifeng Wang et al showed that there was an enhanced resistance of A549 cells to hypoxia induced apoptosis if NDRG2 gene was knocked down by siRNA. It was noticeable that the ectopic expression of NDRG2 only enhanced the apoptosis of A549 cells upon hypoxia, but not under normal culture status.[1] It is unknown if the the expression of NDRG-2 gene is directly responsible for apoptosis of tumour cells or if it is a signal for the apoptosis process NDRG-2 expression is regulated by the Hypoxia-inducible factor (HIF-1) in tumor cell line under hypoxic condition, the Hypoxia responsive element (HRE-1) is one of the three HREs identified in the promoter of NDRG2 gene that bind to the HIF-1 importantly for the expression
The NDRG2 function is to down-regulate the mutated myc gene to inhibit the tumor cell proliferation. NDRG2 is a hypoxia induced gene that functions in apoptosis to kill the tumor cells, in the presence of lower level of oxygen, NDRG2 is more expressed. Hypoxia also induced the translocation of NDRG2 from cytoplasm to nuclear of tumor cells, although the nuclear localisation signal (NLS) as a nuclear import element is not identified in NDRG2 protein, but it’s speculated that NDRG2 protein has its own motif for the nuclear translocation. [1] Lifeng Wang et al showed that there was an enhanced resistance of A549 cells to hypoxia induced apoptosis if NDRG2 gene was knocked down by siRNA. It was noticeable that the ectopic expression of NDRG2 only enhanced the apoptosis of A549 cells upon hypoxia, but not under normal culture status.[1] It is unknown if the the expression of NDRG-2 gene is directly responsible for apoptosis of tumour cells or if it is a signal for the apoptosis process. NDRG2 expression is regulated by the Hypoxia-inducible factor (HIF-1) in tumor cell line under hypoxic condition, the Hypoxia responsive element (HRE-1) is one of the three HREs identified in the promoter of NDRG2 gene that bind to the HIF-1 importantly for the expression .
From the MSA it can be deduced that there is high conservation of NDRG-2 protein throughout many different organisms and it can be concluded that the protein plays a very important role in the biology of a cell. The most highly conserved and closely related branch was the blue branch which had the highest bootstrap values. The purple branch which contained proteins from different species of insects was more closely related to plants than the animals. This is highly interesting and could be a result of poorly optimized tree and low identity to target sequence. The addition of wider range of species including bacteria would confirm the placement of streptomyces lividans in the tree and would help to strength the overall tree.
Conclusion
In conclusion, the NDRG 2 proteins most likely functions as a regulator of H2O2 within many organism which plays a crucial role in maintaining the stability of DNA of cells. The NDRG2 function is also believed to be suppression of tumor cell by inducing the apoptosis for those are subjected to hypoxic condition, the regulation of NDRG2 expression is dependent on the Myc gene expression, which it functions in other DNA binding. The less Myc gene express the more NDRG2 express, when the Myc gene mutate to the oncoprotein then the NDRG2 expression would be no longer suppressed, mutated Myc gene results in abnormal cell differentiation and would lead to the development of tumor cell, the NDRG2 gene would be then expressed since the lower level of Myc gene expression acts as a signal that trigger the NDRG2. This is backed up by the high abundance of NDRG-2 protein in many organism, particularly complex multicellular eukaryotes where regulation at this level is crucial to ensure functionality and stability of the organisms. NDRG-2 is also highly conserved throughout many different mammals, plants, birds and insects which further strengthens this idea. Future directions for the study would require more sequence analysis to identify more homologous proteins to further strength the phylogenetic tree.
Reference
- [1]Lifeng Wang, Na Liu, Libo Yao, Fuyang Li1, Jian Zhang1,Yanchun Deng, Junye Liu, Shaoping Ji, Angang Yang, Hua Han, Yingqi Zhang, Jing Zhang, Wei Han,and Xinping Liu.NDRG2 is a new HIF-1 Target Gene Necessary for Hypoxia-Induced Apoptosis in A549 cells.Cellular Physiology and Biochemistry.Cell Physiol Biochem 2008;21:239-250.
- [2]Jian Zhang, Fuyang Li1, Xinping Liu, Lan Shen, Junye Liu, Jin Su, Wei Zhang, Yanchun Deng, Lifeng Wang, Na Liu, Wei Han, Jing Zhang, Shaoping Ji, Angang Yang, Hua Han, and Libo Yao. The Repression of Human Differentiation-related Gene NDRG2 Expression by Myc via Miz-1-dependent Interaction with the NDRG2 Core Promoter. THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 281, NO. 51, pp. 39159–39168, December 22 2006
- [3]Boulkroun Sheerazed, Fay Michel, Zennaro Maria-Christina, Escoubet Brigitte*, Jaisser Frederic, Blot-Chabaud Marcel, Farman Nicolette and Courtois-Coutry Nathalie. CHARACTERIZATION OF RAT NDRG2 (N-myc Downstream-Regulated Gene 2), A NOVEL EARLY MINERALOCORTICOID-SPECIFIC INDUCED GENE. JBC Papers in Press. Published on June 18, 2002 as Manuscript M200272200
- [4]Staller, P., Peukert, K., Kiermaier, A., Seoane, J. L. J., Karsunky, H., Moroy,T., Bartek, J., Massague, J., and Hanel, F. (2001) Nat. Cell Biol. 3, 392–399
- [5]Kurdistani, S. K., Arizti, P., Reimer, C. L., Sugrue, M. M., Aaronson, S. A., and Lee, S. W. (1998) Cancer Res. 58, 4439–4444
- [6]Eilers, M., Schirm, S., and Bishop, J. M. (1991) EMBO J. 10, 133–141
- [7]Blackwood, E. M., Kretzner, L., and Eisenman, R. N. (1992) Curr. Opin. Genet. Dev. 2, 227–235
- [8]Shachaf, C., Kopelman, A., Arvanitis, C., Karlsson, A., Beer, S., Mandl, S., Bachmann, M. H., Borowsky, A. D., Ruebner, B.,Cardiff, R. D., Yang, Q., Bishop, J. M., Contag, C. H., and Felsher, D. W. (2004) Nature 431, 1112–1117
- [9]Salnikow K, Blagosklonny MV, Ryan H, Johnson R, Costa M: Carcinogenic nickel induces genes involved with hypoxic stress .Cancer Res 2000;60:38-41.
- [10]Semenza GL: Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1.Annu Rev Cell Dev Biol 1999;15:551-578.
- [11]Chen B, Nelson DM, Sadovsky Y: N-myc down-regulated gene 1 modulates the response of term human trophoblasts to hypoxic injury.J Biol Chem 2006;281:2764-2772.
- [12]Sowter HM, Raval RR, Moore JW, Ratcliffe PJ, Harris AL: Predominant role of hypoxia-inducible transcription factor (Hif)-1alpha versusHif-2alpha in regulation of the transcriptional response to hypoxia.Cancer Res 2003;63:6130- 6134.
- [13]Zhang J, Li F, Liu X, Shen L, Liu J, Su J, Zhang W, Deng Y, Wang L, Liu N, Han W, Zhang J, Ji S, Yang A, Han H, Yao L: The repression of human differentiation related gene NDRG2 expression by MYC via MIZ-1 dependent interaction with the NDRG2 core promoter. J Biol Chem 2006;281:39159-39168. http://www.ncbi.nlm.nih.gov/pubmed/7885389
- [14]Lehninger, Principles of Biochemistry, 4th ed. (pp 216-219)