Phytanoyl-CoA dioxygenase Function: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
[[Phytanoyl-CoA dioxygenase domain containing 1 isoform a | Return to main page...]] | [[Phytanoyl-CoA dioxygenase domain containing 1 isoform a | Return to main page...]] | ||
Interpro analysis showed motif similarity to the family of phytanoyl-CoA hydroxylases (PhyH) (''Figure 1'') and Secondary Structure analysis via profunc showed high structural identity to phytanoyl-CoA hydroxylase (PDB code; 2a1x) (''Figure 2''). Although the sequence homology between Phytanoyl-CoA dioxygense and the hydroxylase identified was only 24.9%, a pymol analysis showed the two enzymes to have a high structural similarity. | |||
[[Image:Motif alignment.jpg]] | |||
''Figure 1; A motif alignment from InterPro showing motif similarity between Phytanoyl-CoA dioxygenase and the phytanoyl-CoA hydroxylase (PhyH) family of proteins'' | |||
[[Image:fold analysis.jpg]] | |||
''Figure 2; A fold analysis from Profunc's secondary structure matching program. Note the fold similarity between phytanoyl-CoA hydroxylase (2a1x) and phytanoyl-CoA dioxygenase (Z score 9.5). The two proteins have a 24.9% sequence homology'' | |||
Phytanoyl-CoA hydroxylase is an enzyme catalysing the first step of alpha oxidation in the peroxisome. It functions as an oxidoreductase to oxidise phytanoyl-CoA to 2-hydroxyphytanoyl-CoA. 2-oxoglutarate is simultaniously reduced to succinate and carbon dioxide. | |||
Alpha oxidation is a series of reactions in the peroxisome that remove the alpha carbon from uneven, long-chain fatty acids. The products of alpha oxidation are fed into the peroxisomal beta oxidation pathway before transport to the mitochondria for further beta oxidation to acetyl-CoA. Acetyl-CoA is the entry product into the tricarboxylic acid (TCA) cycle of energy production. | |||
Revision as of 04:04, 3 June 2008
Interpro analysis showed motif similarity to the family of phytanoyl-CoA hydroxylases (PhyH) (Figure 1) and Secondary Structure analysis via profunc showed high structural identity to phytanoyl-CoA hydroxylase (PDB code; 2a1x) (Figure 2). Although the sequence homology between Phytanoyl-CoA dioxygense and the hydroxylase identified was only 24.9%, a pymol analysis showed the two enzymes to have a high structural similarity.
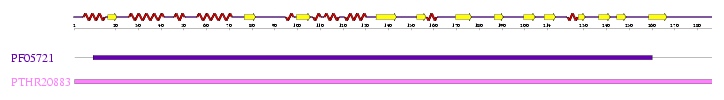 Figure 1; A motif alignment from InterPro showing motif similarity between Phytanoyl-CoA dioxygenase and the phytanoyl-CoA hydroxylase (PhyH) family of proteins
Figure 1; A motif alignment from InterPro showing motif similarity between Phytanoyl-CoA dioxygenase and the phytanoyl-CoA hydroxylase (PhyH) family of proteins
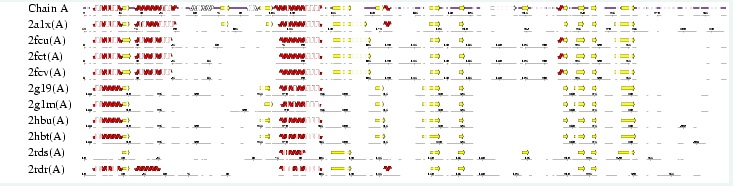 Figure 2; A fold analysis from Profunc's secondary structure matching program. Note the fold similarity between phytanoyl-CoA hydroxylase (2a1x) and phytanoyl-CoA dioxygenase (Z score 9.5). The two proteins have a 24.9% sequence homology
Figure 2; A fold analysis from Profunc's secondary structure matching program. Note the fold similarity between phytanoyl-CoA hydroxylase (2a1x) and phytanoyl-CoA dioxygenase (Z score 9.5). The two proteins have a 24.9% sequence homology
Phytanoyl-CoA hydroxylase is an enzyme catalysing the first step of alpha oxidation in the peroxisome. It functions as an oxidoreductase to oxidise phytanoyl-CoA to 2-hydroxyphytanoyl-CoA. 2-oxoglutarate is simultaniously reduced to succinate and carbon dioxide.
Alpha oxidation is a series of reactions in the peroxisome that remove the alpha carbon from uneven, long-chain fatty acids. The products of alpha oxidation are fed into the peroxisomal beta oxidation pathway before transport to the mitochondria for further beta oxidation to acetyl-CoA. Acetyl-CoA is the entry product into the tricarboxylic acid (TCA) cycle of energy production.