Phytanoyl-CoA dioxygenase Function: Difference between revisions
No edit summary |
No edit summary |
||
| Line 22: | Line 22: | ||
'''Fe2+ binding sites''' | '''Fe2+ binding sites''' | ||
A putative iron binding site was identified in phytanoyl-CoA dioxygenase by structural comparison to the iron binding site of phytanoyl-CoA hydroxylase. Residues PRO155, HIS156, GLN157 and ASP158 show structural similarity to the PRO173, HIS175, GLN176 and ASP177 of the hydroxylase enzyme. HIS220 is also involved in iron binding in the hyroxylase but no histadine residue exist in this position in the dioxygenase. It is hypothesised that the histadine binding is replaced by a serine at position 160, which is spacially close to the histadine in the pymol alignment. | A putative iron binding site was identified in phytanoyl-CoA dioxygenase by structural comparison to the iron binding site of phytanoyl-CoA hydroxylase. Residues PRO155, HIS156, GLN157 and ASP158 show structural similarity to the PRO173, HIS175, GLN176 and ASP177 of the hydroxylase enzyme. HIS220 is also involved in iron binding in the hyroxylase but no histadine residue exist in this position in the dioxygenase. It is hypothesised that the histadine binding is replaced by a serine at position 160, which is spacially close to the histadine in the pymol alignment. | ||
Revision as of 04:08, 3 June 2008
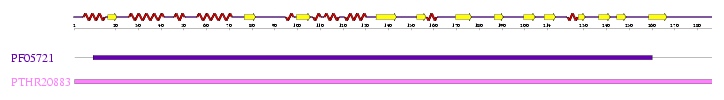
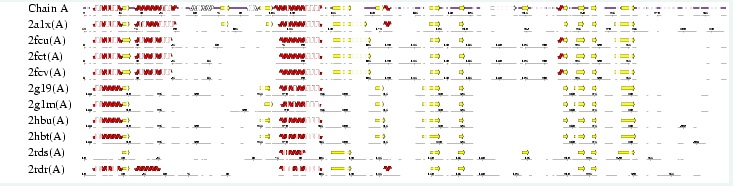
Interpro analysis showed motif similarity to the family of phytanoyl-CoA hydroxylases (PhyH) (Figure 1) and Secondary Structure analysis via profunc showed high structural identity to phytanoyl-CoA hydroxylase (PDB code; 2a1x) (Figure 2). Although the sequence homology between Phytanoyl-CoA dioxygense and the hydroxylase identified was only 24.9%, a pymol analysis showed the two enzymes to have a high structural similarity.
Figure 1; A motif alignment from InterPro showing motif similarity between Phytanoyl-CoA dioxygenase and the phytanoyl-CoA hydroxylase (PhyH) family of proteins
Figure 2; A fold analysis from Profunc's secondary structure matching program. Note the fold similarity between phytanoyl-CoA hydroxylase (2a1x) and phytanoyl-CoA dioxygenase (Z score 9.5). The two proteins have a 24.9% sequence homology
Phytanoyl-CoA hydroxylase
Phytanoyl-CoA hydroxylase is an enzyme catalysing the first step of alpha oxidation in the peroxisome. It functions as an oxidoreductase to oxidise phytanoyl-CoA to 2-hydroxyphytanoyl-CoA. 2-oxoglutarate is simultaniously reduced to succinate and carbon dioxide.
Alpha oxidation is a series of reactions in the peroxisome that remove the alpha carbon from uneven, long-chain fatty acids. The products of alpha oxidation are fed into the peroxisomal beta oxidation pathway before transport to the mitochondria for further beta oxidation to acetyl-CoA. Acetyl-CoA is the entry product into the tricarboxylic acid (TCA) cycle of energy production.
Pymol analysis of similarity between Phytanoyl-CoA dioxygenase and Phytanoyl-CoA hydroxylase
Inferance of protein function is made by comparison of structure between phytanoyl-CoA dioxygenase and phytanoyl-CoA hydroxylase. Although not sequentially similar (Blast score? Clustalx score?) a pymol alignment highlights the structural similarities.
Fe2+ binding sites
A putative iron binding site was identified in phytanoyl-CoA dioxygenase by structural comparison to the iron binding site of phytanoyl-CoA hydroxylase. Residues PRO155, HIS156, GLN157 and ASP158 show structural similarity to the PRO173, HIS175, GLN176 and ASP177 of the hydroxylase enzyme. HIS220 is also involved in iron binding in the hyroxylase but no histadine residue exist in this position in the dioxygenase. It is hypothesised that the histadine binding is replaced by a serine at position 160, which is spacially close to the histadine in the pymol alignment.