Pyridoxal Phosphatase Introduction
An Introduction to Protein Phosphatases
The biological significance of protein phosphatases - their structures, rather - is the information provided pertaining to both normal and pathophysiologic processes, including the regulation of transcription and major signalling pathways and diseases such as type 1 diabetes. These studies could contribute significantly towards pathogenesis studies, and ultimately bring cellular and molecular biology studies to a whole new level (Almo et al., 2007).
The structures of protein phosphatases, in general, consists of a catalytic domain comprising of a beta sheet sandwiched between alpha helices. The active site revolves around the magnesium ion (in the case of 2cfsA, there are 2 Mg ions) which is involved in the phosphate-cleaving reaction. In the case of Pyridoxal Phosphatase, an additional large domain covers the active site, creating tighter active sites, a feature typical of phosphatases that act on small molecules. The purpose of these large domains are to create tighter active sites (Almo et al., 2007)
There are 2 main families of protein phosphatases, namely the protein tyrosine phosphatases (PTPs) - which themselves have 4 sub-families; and the serine/threonine protein phosphatases, which are represented by 2 sub-families. For this paper, evidence suggests that 2cfsA belongs to the family of serine/threonine protein phosphatases. This sub-family is the PPM, or PP2C-like family, where members are Mn/Mg-dependent enzymes. In the structural component of this paper, it will be easy to understand why 2cfsA has been hypothesised to be part of this sub-family (HINT: take note of the type of metal ions possessed by 2cfsA). Members of the serine/threonine protein phosphatase family may not necessarily have homology in terms of their sequences, but the key lies in the catalytic mechanisms involving their respective metal ions.
Chronophin
Chronophin is a phosphatase (belonging to the Haloacid Dehalogenase, otherwise known as the HAD superfamily) primarily involved in two different biological actions:
- Regulation of the actin cytoskeleton
- Vitamin B6 metabolism
In the context of Vitamin B6 metabolism, Chronophin undertakes this function under the name of Pyridoxal Phosphatase. Chronophin's main role in Vitamin B6 metabolism is the phosphorylation of pyridoxal-5'-phosphate (PLP, or the coenzymatically active form of vitamin B6), and this is part of a complicated pathway for degradation of the vitamin.
Almo et al., 2007 reveals that the PDB code for Chronophin is 2oyc. This PDB code will be appearing rather regularly in the structural component of this paper. Of similar importance is the PLP-bound form of Chronophin, which is obtained by substituting the Magnesium ions for catalytically inert Calcium ions. This affects the catalyic aspartic acid (Asp25), resulting in compete inactivation of the enzyme. An example of the PLP-bound form of Chronophin is also known as Pyridoxal-5'-Phosphate Phosphatase (PDB ID: 2cft), which like 2oyc, will be mentioned further down the paper. (Almo et al., 2007)
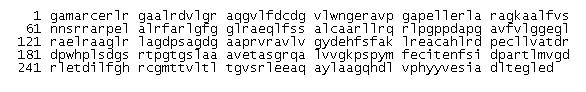
Sequence of 2cfsA and its human homolog
Sequence for the protein of interest, Pyridoxal Phosphatase
Sequence for the human homolog of Pyridoxal Phosphatase