Roles of fascin in motility and invasion
Two new biological contexts where fascin participates in motility and invasion have recently emerged: the movement of intracellular pathogenic bacteria and the clinical aggressiveness of human carcinomas.
Bacterial intracellular motility
Rickettsia conorii is an intracellular pathogen responsible for human spotted fever that targets non-phagocytic cells. Once inside a host cell, the bacteria assemble actin tails on their surfaces that enable movement through the cytoplasm and the generation of membrane protrusions that facilitate bacterial entry into neighboring cells.
Unlike other motile intracellular bacterial pathogens such as Listeria monocytogenes, the actin tails of R. conorii consist entirely of long, unbranched actin filaments. R. conorii induces actin polymerization by a novel mechanism dependent on a bacterial surface protein, RickA, which activates host cell Arp2/3 complex. Very interestingly, fascin is present in the actin tails and is likely to be responsible for the bundling of actin filaments. By analogy with the cellular regulation of fascin protrusions, it seems possible that an additional mechanism could be involved to specify recruitment of fascin to the tail. Indeed, new data on the formation of actin tails by L. monocytogenes have revealed an Arp2/3-independent elongation phase that supports rapid bacterial propulsion and that depends on a distinct set of biochemical factors in addition to those required for initial Arp2/3-dependent motility. In this phase, fascin (or the other actin-bundling proteins fimbrin, a-actinin or filamin) in combination with actin was sufficient to assemble a bundled form of the actin tail that supported fast propulsion (Adams, 2004).
Carcinoma motility and invasion
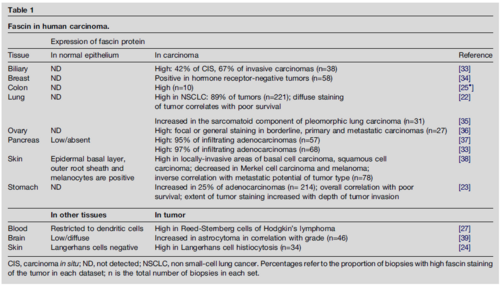
With the exception of the basal layer of the epidermis, fascin has not been reported in normal adult epithelia (Table 1). Recent publications document upregulation of fascin protein in human carcinomas from many body sites (Table 1). In studies of gastric adenocarcinoma, high fascin expression within the tumor correlated with poor survival. Increased fascin content has also been documented in other invasive tumor types such as high-grade astrocytoma (Table 1). The data on carcinomas implicate fascin in the progression to an invasive phenotype and raise the possibility that fascin could be of value as a novel prognostic marker for early identification of aggressive carcinomas. Stable expression of fascin-1 in SW1222 cells, a fascin-negative human colonic epithelial cell line, resulted in increased motility on laminin and increased three-dimensional haptotaxis through collagen-IV-coated filters. Although cell–ECM attachment properties (a measure of integrin ligand-binding activity) and cell-surface expression of b1 integrins were not affected, integrin b1 subunit and vinculin became relocated to the outer leading edges of the fascin-expressing cells. Adherens junctions or the association of E-cadherin with b-catenin were not detectably affected. These results suggest a model in which expression of fascin in colonic epithelial cells activates local motility and/or invasion principally by altering the organization of actin-based protrusions and focal ECM adhesions. Systematic studies of the relationship between alterations in the ECM microenvironment of the tumor and upregulation of fascin are also now needed to assist understanding of the mechanism(s) by which de novo expression of fascin in epithelial cells contributes to the clinical aggressiveness of tumors. In a recent study, it was found that up-regulation of fascin-1 in human carcinomas has a multi-factorial basis and identifying the binding motifs cAMP response element-binding protein (CREB) and aryl hydrocarbon receptor (AhR) as major, specific regulators of FSCN-1 transcription in human carcinoma cells (Hashimoto, Loftis & Adams, 2009).