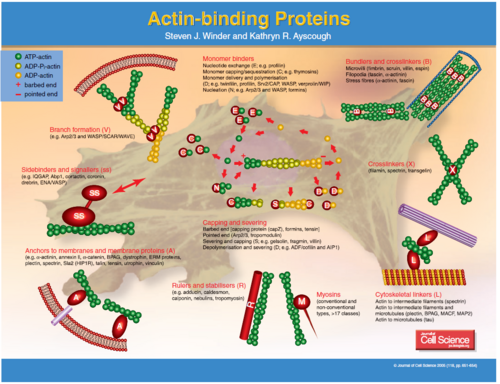
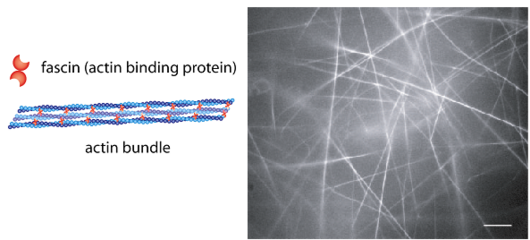
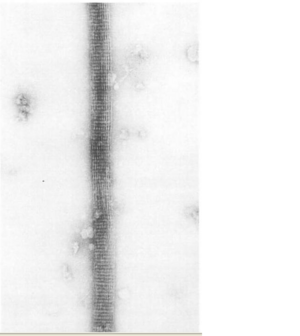
Fascin Function
The fascins are a structurally unique and evolutionarily conserved group of actin cross-linking proteins. They are a 54-58Kda protein that was originally isolated from sea urchin egg (Strongylocentrotus purpuratus) but is also found in drosophila and vertebrates including man. Fascins function in the organisation of two major forms of actin-based structures: dynamic, cortical cell protrusions and cytoplasmic microfilament bundles. Cell protrusions are extensions of the plasma membrane that are supported internally by actin-based structures that impart mechanical stiffness. The cortical structures, which include filopodia, spikes, lamellipodial ribs, oocyte microvilli and the dendrites of dendritic cells, have roles in cell-matrix adhesion, cell interactions and cell migration, whereas the cytoplasmic actin bundles appear to participate in cell architecture and intracellular movements [1]. The fascin–actin interaction is under complex regulation from the extracellular matrix, peptide factors and other actin-binding proteins.
The ultrastructure of actin-fascin needles was described by Kane (1975, 1976) using optical diffraction and image-reconstruction methods. Each needle consisted of a bundle of fascicle with approximate parallel actin filaments arranged in a hexagonal arrangement with 8.3nm spacing. It was found from this that the links in the actin-fascin needle are not evenly spaced along the monomer but occur alternatingly at every 4 and 5 actins (Bryan & Kane, 1978).
Fascin Movement
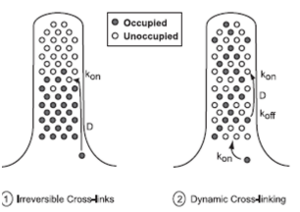
To reach the growing (barbed) end of nascent actin filaments, fascin must be translocated from the cell body into the filopodium and out to the tip. This occurs when fascin is in the phosphorylated state making it primarily freely diffusing, whereas actin bundling in filopodia is accomplished by fascin dephosphorylated at serine 39 (figure 4). However, when filopodia reach long lengths or elongate rapidly, diffusion alone may not be a sufficient mechanism for protein transport. Thus, a process which involves diffusion and rapid, irreversible association can supply fascin only to short filopodia, but dynamic cross-linking is required to sustain longer filopodia.In significantly longer filopodia with irreversible cross-links, diffusing fascin would permanently fill binding spots nearest the base first, and an elongation rate that exceeds fascin supply would leave the tips very low in fascin. Diffusion with dynamic cross-linking might permit a sufficient supply rate to the tip region at longer lengths, by allowing dissociation of bound fascin and continued diffusion and redistribution along the length of the filopodium (figure 5).
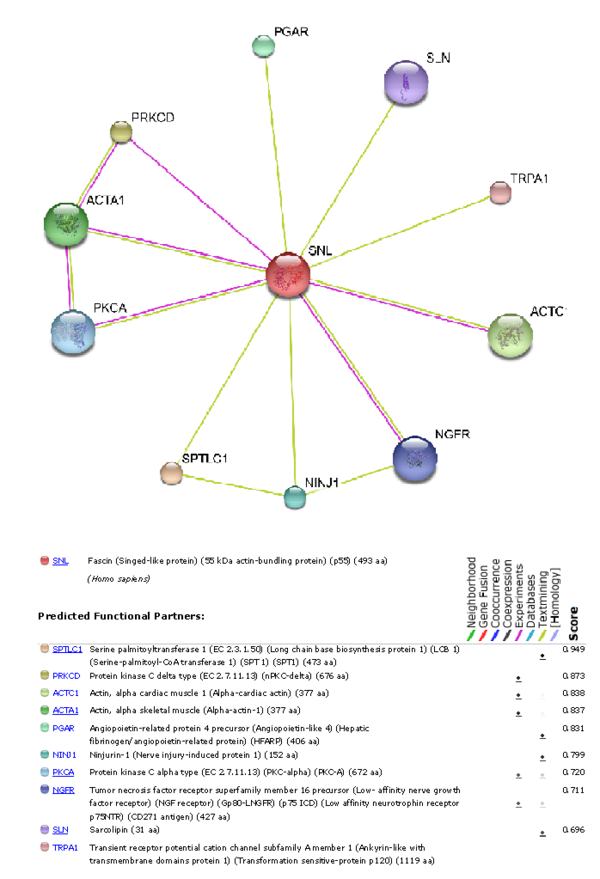
To understand which other proteins Fascin-1 interacts with, using the STRING 8.0 database was used to produce a graph showing which other functional proteins it is predicted to interact with.
Cellular regulation of fascin protrusions