COASY intro
Coenzyme A (CoA) is an essential cofactor, which carries acyl and acytl groups (Zhyvoloup et. al., 2002). It is involved in many cellular processes, including the tricarboxylic acid cycle and fatty acid synthesis and β-oxidation (Zhyvoloup et. al., 2003). It has been estimated that 4% of cellular enzymes require CoA, highlighting its key role (Daugherty et. al., 2002). Changes in CoA levels are associated with several compromised metabolic states and diseases, including starvation, alcoholism, hypertension, diabetes and some tumours (Zhyvoloup et. al., 2002).
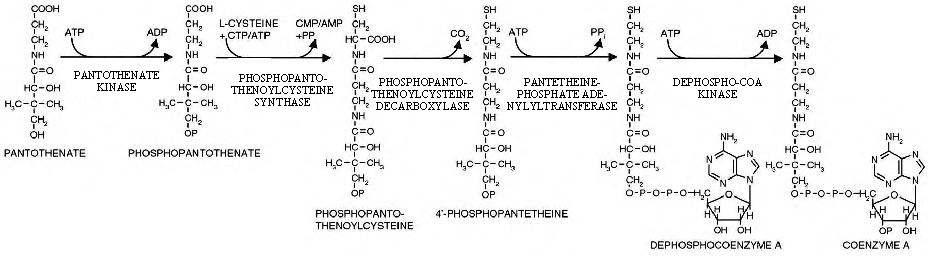
CoA is synthesised from pantothenate (Vitamin B5) in a five-step pathway (Zhyvoloup et. al., 2003). This pathway is used by both prokaryotes and eukaryotes, though the structure of some of the enzymes involved varies between species (Zhyvoloup et. al., 2002). In the first step in CoA synthesis, pantothenate kinase converts pantothenate to 4’phosphopantothenate. This is a rate limiting step (Zhyvoloup et. al., 2002). Secondly, 4’phosphopantothenate is converted to 4’-phosphopantothenolycysteine by phophopantothenoylcysteine synthase. This is then changed to 4’phosphopantetheine by phosphopantothenoylcysteine decarboxylase. In the fourth step, pantetheine-phosphate adenylyltransferase (PPAT) converts 4’phosphopantetheine to dephospho-CoA. Finally, this is converted to CoA by dephospho-CoA kinase (DPCK). This pathway requires ATP and cysteine, and is shown in Figure 1.
In prokaryotes, the enzymes in this pathway exist as distinct proteins. However, in eukaryotes, the final two enzymes of the pathway (PPAT and DPCK) are present in a single bifunctional enzyme, CoA synthase (CoAsy). In mice, this is a 563 amino acid protein which is encoded by the Ukr1 gene, located on chromosome 11 (Zhyvoloup et. al., 2002; RCSB, 2007). Bioinformatic and experimental analysis of the Ukr1 gene product has confirmed that it possesses both PPAT and DPCK domains, and can mediate the final two steps of CoA synthesis (Zhyvoloup et. al., 2002).
The crystal structure of part of this protein (chain A) has recently been determined (PDB code: 2f6rA). This chain is 281 amino acids long, and contains a break after amino acid 25. After initially comparing the sequence of CoA in different species and investigating the relationship between its expression and function, we analysed this structure, and using bioinformatics analysis determined that it contains a DPCK domain and part of a PPAT domain. Most of the PPAT domain, including its ligand binding sites, appears to be missing from the structure, being located in the 302 amino acid sequence that was cut out of the protein during structural determination. However, the DPCK domain was present in the structure, and putative binding sites for ATP and dephospho-CoA on this domain were identified.
Abstract | Introduction | Results | Discussion | Conclusion | Method | References