Fascin Results: Difference between revisions
No edit summary |
No edit summary |
||
| (5 intermediate revisions by 2 users not shown) | |||
| Line 3: | Line 3: | ||
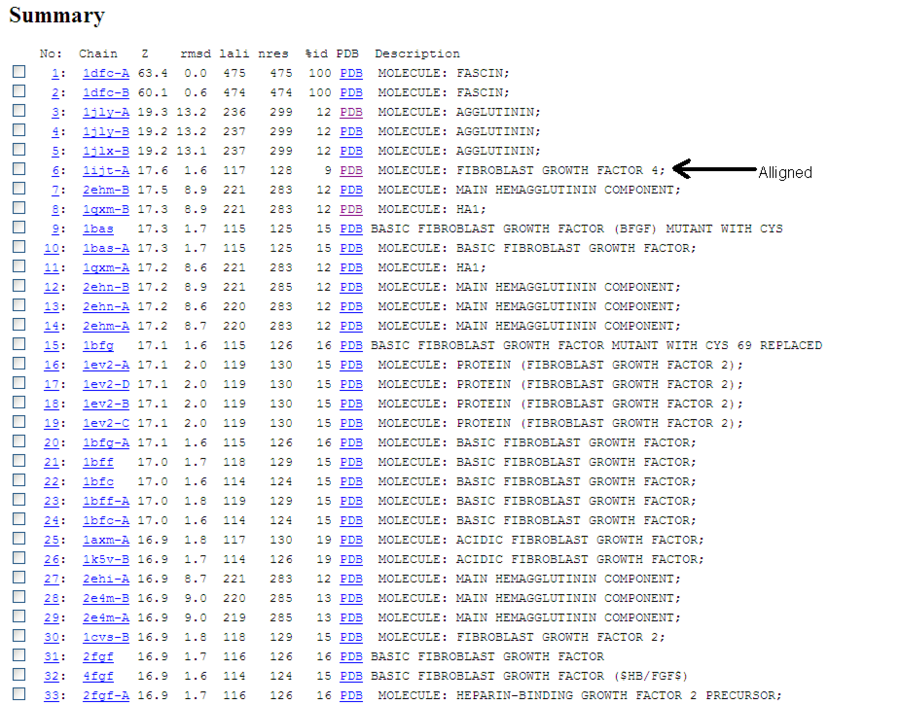
[[Image:Flascin_1.jpg]] | [[Image:Flascin_1.jpg|centre|thumb|800px|'''Figure 1''':Fascin allignment showing identity to several other homologous proteins]] | ||
Sample Fascin sequence (Text File) | Sample Fascin sequence (Text File) | ||
| Line 17: | Line 17: | ||
From a possible 100 FASTA sequences, were cut down to 31. The process of elimination was conducted by the deletion of sequences with too many bp before and after the sample sequence, followed by the deletion of sequences with large segments of gaps in conserved and variable regions of the sequence alignment. Fascin domains were found via a protDOM search and compared to the remaining sequences. | From a possible 100 FASTA sequences, were cut down to 31. The process of elimination was conducted by the deletion of sequences with too many bp before and after the sample sequence, followed by the deletion of sequences with large segments of gaps in conserved and variable regions of the sequence alignment. Fascin domains were found via a protDOM search and compared to the remaining sequences. | ||
[[Image:Fascin_multiple_alignment.jpg]] | [[Image:Fascin_multiple_alignment.jpg|centre|thumb|900px|'''Figure 2''':Multiple allignment]] | ||
| Line 23: | Line 23: | ||
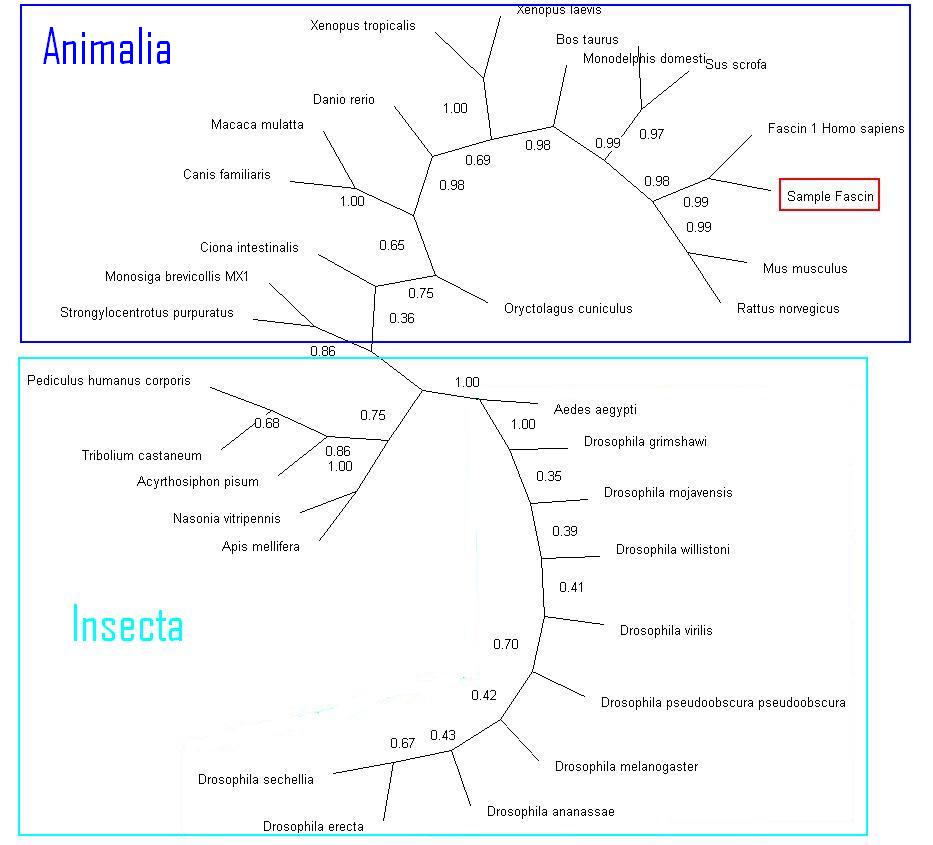
Before any deletion of sequences occured, A tree was formed using 100-replications Dayhoff neighbor-joining method as a base line. | Before any deletion of sequences occured, A tree was formed using 100-replications Dayhoff neighbor-joining method as a base line. | ||
[[Image:Facscin_broad_bootstrap.jpg]] | [[Image:Facscin_broad_bootstrap.jpg|centre|thumb|1000px|'''Figure 3''':Non-specific phylogeny tree encompasing many species]] | ||
Neighbor-method with Bootstrap values (1 = 100) Results were run at 500 replications | Neighbor-method with Bootstrap values (1 = 100) Results were run at 500 replications | ||
[[Image:Fascin_bootstrap_500rep.jpg]] | [[Image:Fascin_bootstrap_500rep.jpg|centre|thumb|1000px|'''Figure 4''':Neighbor-method phylogeny tree]] | ||
Neighbor-method with Bootstrap values, compressed. Sequences with below 50 were deleted. | Neighbor-method with Bootstrap values, compressed. Sequences with below 50 were deleted. | ||
[[Image:Fascin_bootstrap_animaliainsecta.jpg]] | [[Image:Fascin_bootstrap_animaliainsecta.jpg|centre|thumb|1000px|'''Figure 5''': Specific phylogeny tree of classes insecta and anamalia]] | ||
== Fascin structure and domains == | == Fascin structure and domains == | ||
| Line 39: | Line 39: | ||
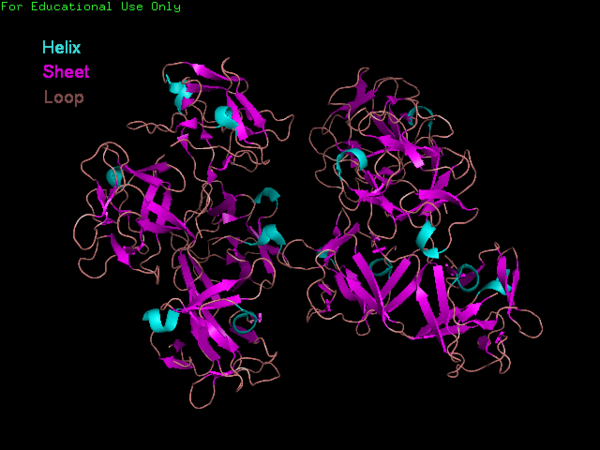
The Fascin protein is comprised of 8 repeats of the Fascin-like domain in total. It is made of two identical subgroups, each of which contains 4 Fascin-like domains linked together. The 2 subgroups are linked back to back slightly off-centre. The Fascin-like domain's tertiary structure adopts a beta-trefoil fold pattern with an internal threefold repeat. Secondary structure is predominantly Beta-sheet and random coil (loop) with small regions of alpha-helix. Structural similarity with several unrelated proteins is evident (eg. FGF). | The Fascin protein is comprised of 8 repeats of the Fascin-like domain in total. It is made of two identical subgroups, each of which contains 4 Fascin-like domains linked together. The 2 subgroups are linked back to back slightly off-centre. The Fascin-like domain's tertiary structure adopts a beta-trefoil fold pattern with an internal threefold repeat. Secondary structure is predominantly Beta-sheet and random coil (loop) with small regions of alpha-helix. Structural similarity with several unrelated proteins is evident (eg. FGF). | ||
[[Image:Fascin sec struc and domain.PNG|centre|thumb|800px|'''Figure | [[Image:Fascin sec struc and domain.PNG|centre|thumb|800px|'''Figure 6''': Layout of primary and secondary sequences with domain annotations. (Finn et. al. 2006, Kabsch et. al. 1983, Murzin et. al. 2005)]] | ||
[[Image:Fascin secondary structure.png|centre|thumb|600px|'''Figure | [[Image:Fascin secondary structure.png|centre|thumb|600px|'''Figure 7''': 'Front' view showing secondary structure of Fascin.]] | ||
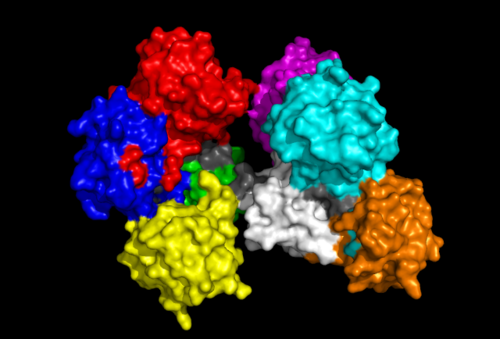
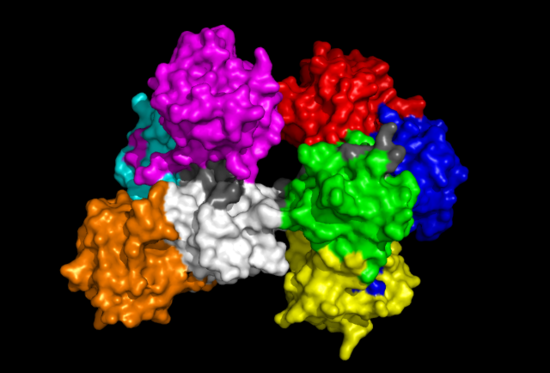
[[Image:Fascin domains and conserved.png|left|thumb|500px|'''Figure | [[Image:Fascin domains and conserved.png|left|thumb|500px|'''Figure 8''': 'Front' view of Fascin protein with each Fascin domain coloured. Red, Blue, Green and Yellow segments represent one subgroup while Aqua, Magenta, White and Orange represent the other. Grey colouration represents conserved residues found in sequence allignment.]] | ||
[[Image:Fascin domains and conserved back.png|right|thumb|550px|'''Figure | [[Image:Fascin domains and conserved back.png|right|thumb|550px|'''Figure 9''': 'Back' view of Fascin protein. Same colouration as above.]] | ||
| Line 55: | Line 55: | ||
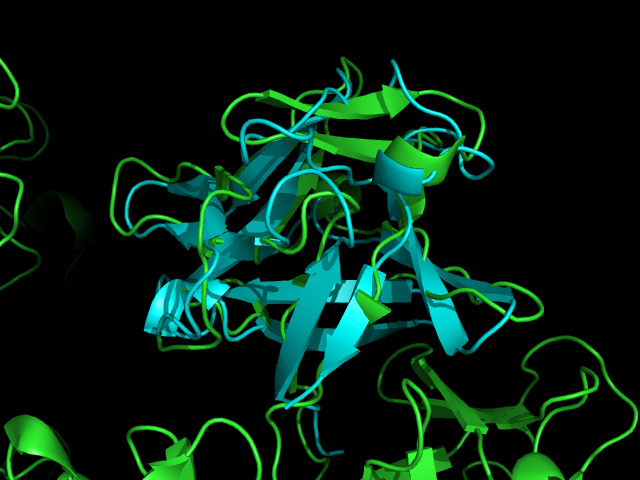
[[Image:Fascin dali.PNG|centre|thumb|900px|'''Figure | [[Image:Fascin dali.PNG|centre|thumb|900px|'''Figure 10''': Dali output showing first 33 structurally similar protein results.]] | ||
[[Image:Structural comparrison Fascin FGF.png|centre|thumb|700px|'''Figure | [[Image:Structural comparrison Fascin FGF.png|centre|thumb|700px|'''Figure 11''': Pymol allignment of Fascin 1 (green) and FGF (aqua)]] | ||
Similarity is between FGF and the Fascin domain (not the molecule). | Similarity is between FGF and the Fascin domain (not the molecule). | ||
| Line 67: | Line 67: | ||
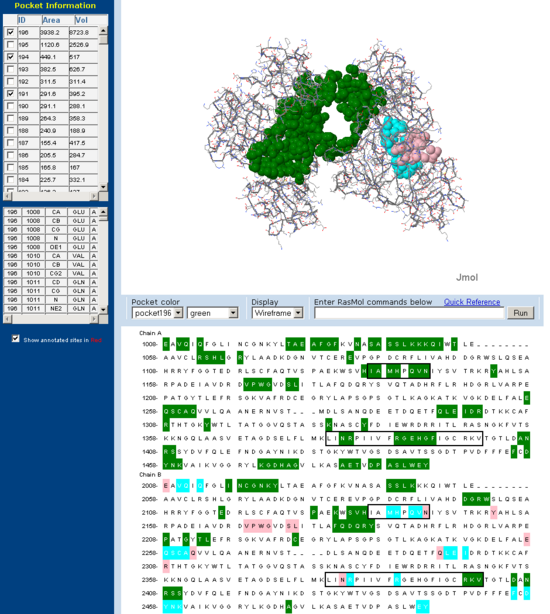
[[Image:Fascin CASTp front.PNG|left|thumb|550px|'''Figure | [[Image:Fascin CASTp front.PNG|left|thumb|550px|'''Figure 12''': Putative actin binding pocket regions on Fascin 'front view'.]] | ||
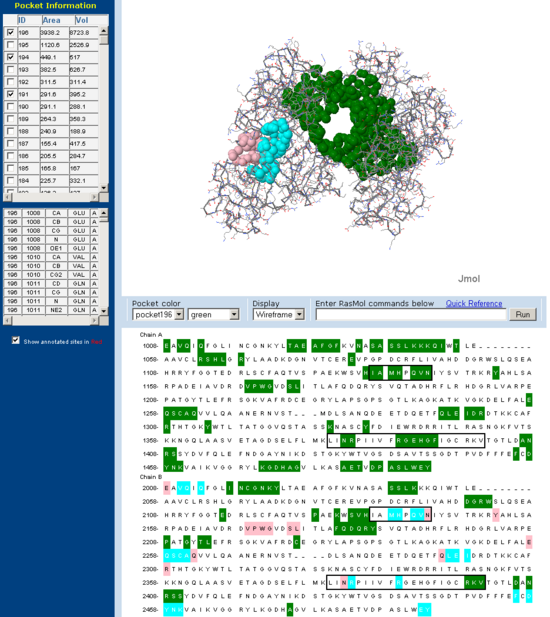
[[Image:Fascin CASTp back.PNG|right|thumb|550px|'''Figure | [[Image:Fascin CASTp back.PNG|right|thumb|550px|'''Figure 13''': Putative actin binding pocket regions on Fascin 'back view'.]] | ||
| Line 75: | Line 75: | ||
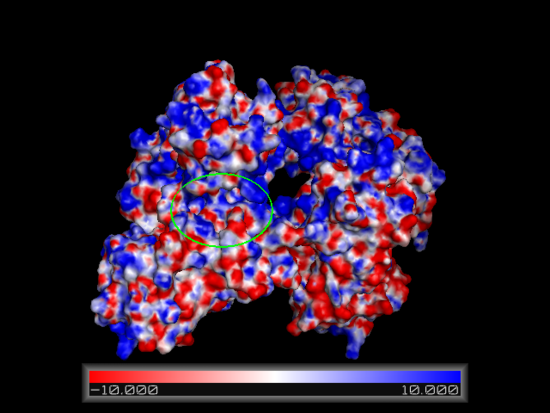
Using the online PDB2PQR resource in conjunction with the APBS feature in pymol an electrostatic surface model for Fascin was generated. Regions corresponding to our putative actin binding sites were circled. Note the positively charged regions encapsulating a negatively charged centre. | Using the online PDB2PQR resource in conjunction with the APBS feature in pymol an electrostatic surface model for Fascin was generated. Regions corresponding to our putative actin binding sites were circled. Note the positively charged regions encapsulating a negatively charged centre. | ||
[[Image:Fascin electro front binding.PNG|left|thumb|550px|'''Figure | [[Image:Fascin electro front binding.PNG|left|thumb|550px|'''Figure 14''': Front view of electrostatic surface model. Putative actin binding site 1 (front) circled in green.]] | ||
[[Image:Fascin electro back binding.PNG|right|thumb|550px|'''Figure | [[Image:Fascin electro back binding.PNG|right|thumb|550px|'''Figure 15''': Back view of electrostatic surface model. Putative actin binding site 2 (back) circled in green.]] | ||
== Hydrophobicity Plot == | == Hydrophobicity Plot == | ||
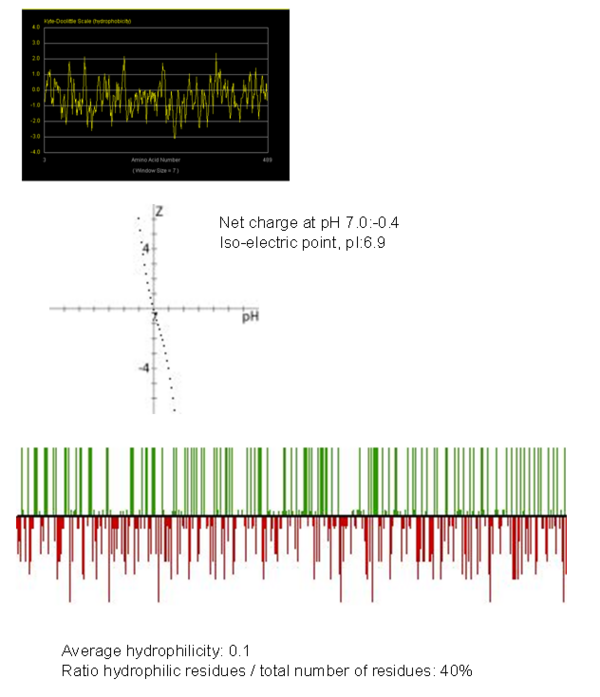
[[image:Untitled10.PNG|centre|thumb|600px|'''Figure | A hydrophobicity plot was generated and showed that overall the Fascin-1 protein is hydrophobic. | ||
[[image:Untitled10.PNG|centre|thumb|600px|'''Figure 16''': Plot showing the hydrophobicity of fascin-1 protein and second plot showing hydrophilicty plot which acts as a peptide property calculator. From these results we can overall state that this protein overall is hydrophobic.]] | |||
== Important molecular regions == | == Important molecular regions == | ||
| Line 88: | Line 90: | ||
The image below shows Fascin's overall composition and these 2 important regions. | The image below shows Fascin's overall composition and these 2 important regions. | ||
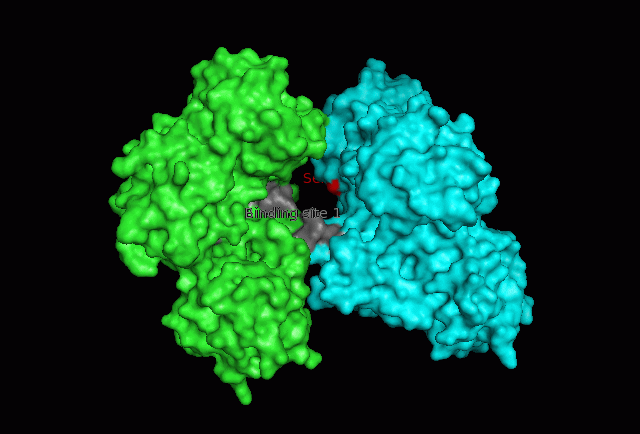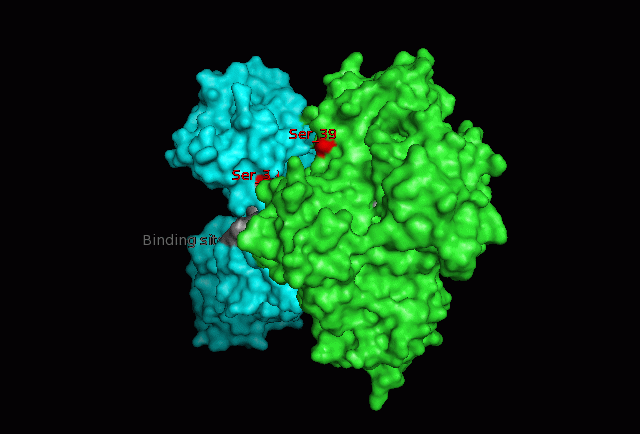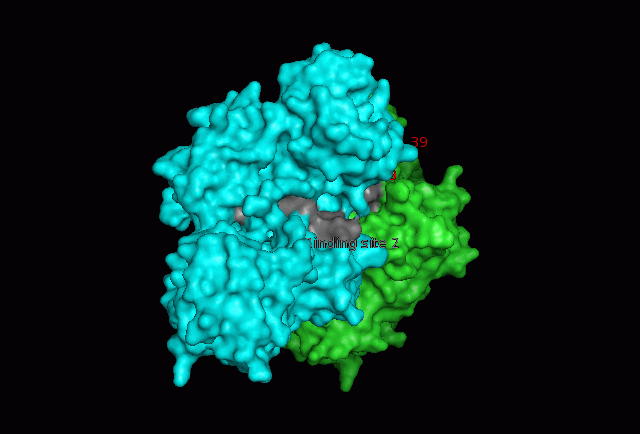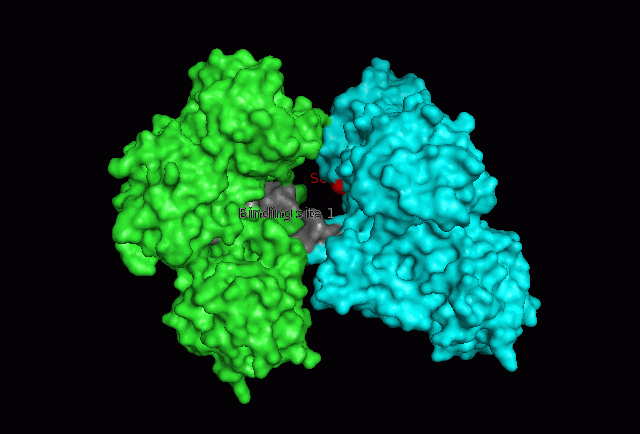
[[Image:Fascin movie.gif|centre|thumb|700px|'''Figure | [[Image:Fascin movie.gif|centre|thumb|700px|'''Figure 17''': 360 degree view of Fascin. Putatitive front (1) and back (2) actin binding sites are shown in grey and Serine 39 residues on both Fascin subgroups are shown in red.]] | ||
Putative actin binding sites were localised to residues 136-143 and 386-395. | |||
== Protein Interaction and Cellular Location == | |||
Interaction of other proteins with Fascin-1 and its concentrations levels in different regions of the body were analysed and represented below. | |||
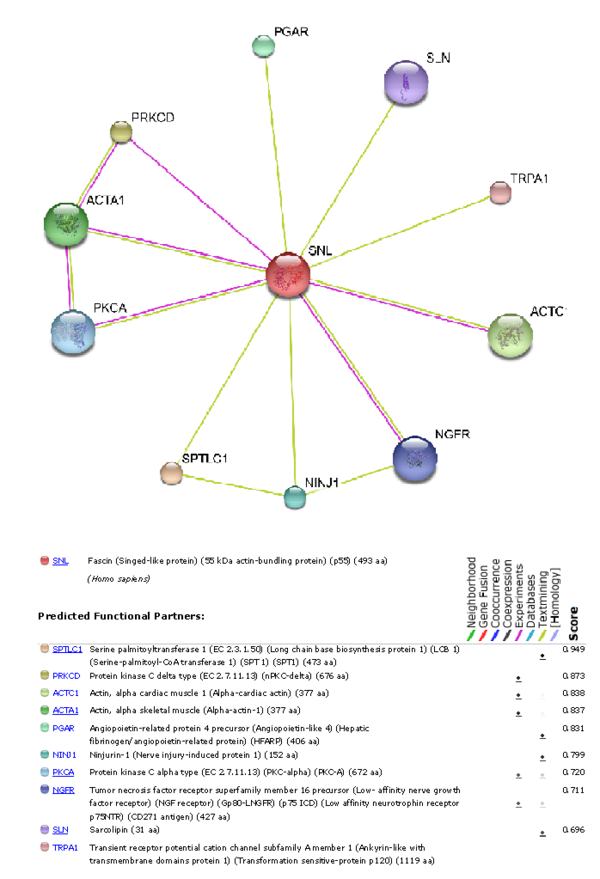
[[image:Untitled7.PNG|centre|thumb|600px|'''Figure 18''': Image showing various predicted functional partners that interact with Fascin-1.]] | |||
[[Image: Untitled8.PNG|centre|thumb|600px|'''Figure 19''': Image showing expression in various human cell types]] | |||
[[Fascin Abstract | Abstract]] | [[Fascin Introduction | Introduction]] | [[Fascin Methods | Methods]] | [[Fascin Results | Results]] | [[Fascin Discussion | Discussion]] | [[Fascin Conclusion | Conclusion | [[Fascin Abstract | Abstract]] | [[Fascin Introduction | Introduction]] | [[Fascin Methods | Methods]] | [[Fascin Results | Results]] | [[Fascin Discussion | Discussion]] | [[Fascin Conclusion | Conclusion]] | [[Fascin References | References]] | ||
<br>[[Fascin 1 | Back to main page]] | <br>[[Fascin 1 | Back to main page]] | ||
Latest revision as of 02:01, 16 June 2009
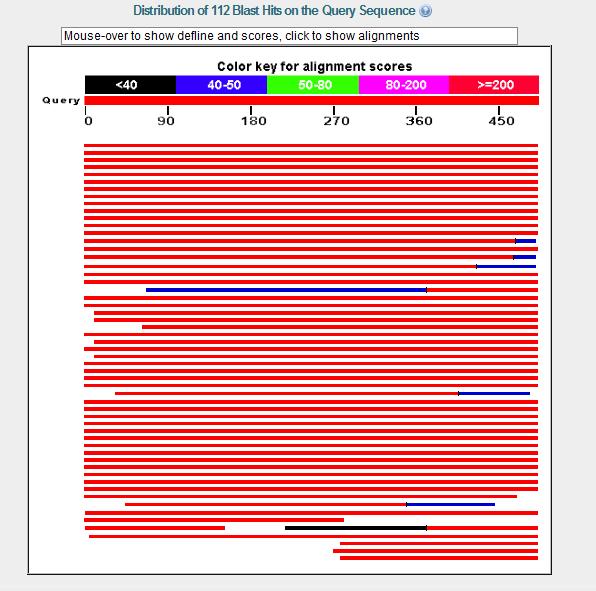
BLAST
Blast Results show that fascin 1 is very highly conserved throughout the Eukarya Domain providing 112 hits all with excellent E value and High scores
Sample Fascin sequence (Text File)
FASTA sequences from BLAST (Text File)
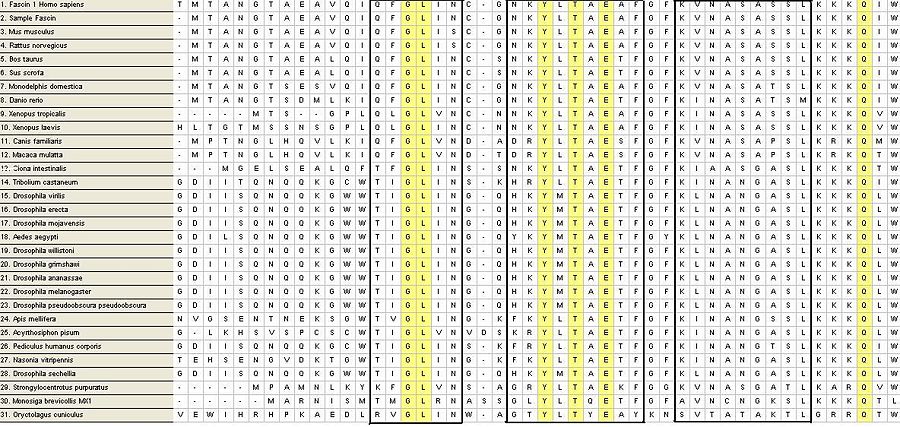
Multiple Alignment
From a possible 100 FASTA sequences, were cut down to 31. The process of elimination was conducted by the deletion of sequences with too many bp before and after the sample sequence, followed by the deletion of sequences with large segments of gaps in conserved and variable regions of the sequence alignment. Fascin domains were found via a protDOM search and compared to the remaining sequences.
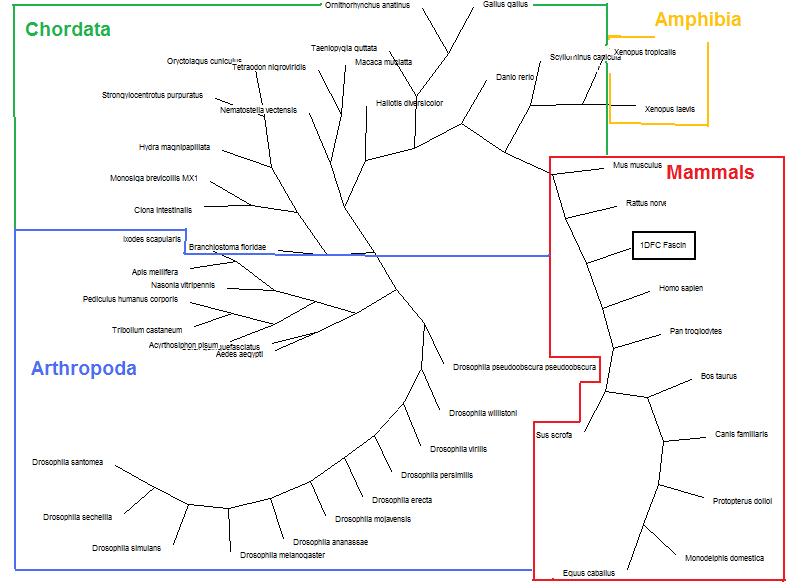
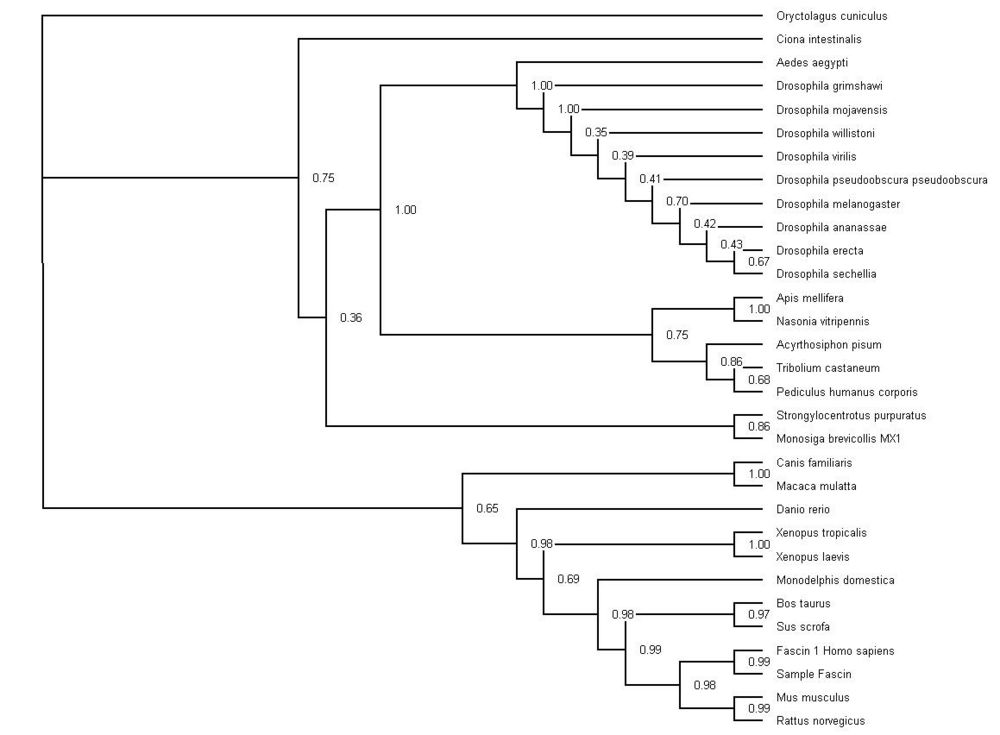
Phylogeny Trees
Before any deletion of sequences occured, A tree was formed using 100-replications Dayhoff neighbor-joining method as a base line.
Neighbor-method with Bootstrap values (1 = 100) Results were run at 500 replications
Neighbor-method with Bootstrap values, compressed. Sequences with below 50 were deleted.
Fascin structure and domains
The Fascin protein is comprised of 8 repeats of the Fascin-like domain in total. It is made of two identical subgroups, each of which contains 4 Fascin-like domains linked together. The 2 subgroups are linked back to back slightly off-centre. The Fascin-like domain's tertiary structure adopts a beta-trefoil fold pattern with an internal threefold repeat. Secondary structure is predominantly Beta-sheet and random coil (loop) with small regions of alpha-helix. Structural similarity with several unrelated proteins is evident (eg. FGF).
Structual allignments
A Dali database search returned several hundred similar structures to Fascin 1. The first page of results can be seen below. Unfortunately none of the structurally similar molecules were actin binding so could not help with actin binding regions. The molecule 1ijt (Fibroblast Growth Factor - FGF) was alligned with Fascin to show structural similarity (even though they are functionally very different and only contain 9% sequence identity). Structurally FGF has much more Beta sheet secondarty structure, but as said before they are still quite similar.
Similarity is between FGF and the Fascin domain (not the molecule).
Cleft/Pocket analysis
Pockets and clefts in the molecular surface of Fascin were analysed using the online service CASTp (Dundas J et. al. 2006). Three major pockets corresponding to the beleived actin binding sites for Fascin are shown with both a front and back view. Conserved regions from 'across species' multiple sequence allignment are outlined in the sequence window. Note how many of the residues involved in the pocket are from these conserved regions.
Electrostatic surface model
Using the online PDB2PQR resource in conjunction with the APBS feature in pymol an electrostatic surface model for Fascin was generated. Regions corresponding to our putative actin binding sites were circled. Note the positively charged regions encapsulating a negatively charged centre.
Hydrophobicity Plot
A hydrophobicity plot was generated and showed that overall the Fascin-1 protein is hydrophobic.
Important molecular regions
The focus so far has been on binding of Fascin to its ligand actin. Fascin actually interacts with many other protein molecules to undergo its function. One such molecule is the phosphorylating molecule phosphokinase-C (PKC). Phosphorylation of serine residues 39 on a Fascin subgroup alters the actin binding properties of the molecule. From our research we have also found that although it is known Fascin binds actin, the site of binding is still unknown. Through multiple sequence allignment across species, electrostatic modeling and pocket analysis we have tried to determine the actin binding site on the Fascin molecule. The image below shows Fascin's overall composition and these 2 important regions.
Putative actin binding sites were localised to residues 136-143 and 386-395.
Protein Interaction and Cellular Location
Interaction of other proteins with Fascin-1 and its concentrations levels in different regions of the body were analysed and represented below.
Abstract | Introduction | Methods | Results | Discussion | Conclusion | References
Back to main page