LOC56985 Structure Main
Structure Comparison
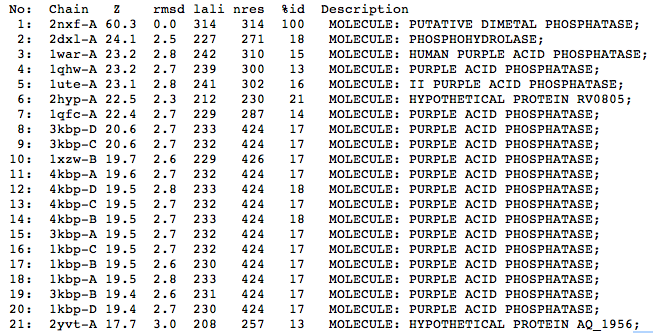
The Dali results indicate that structurally the closest relative to the protein is a phosphohydrolase (2DXL) from Enterbacter aerogenes. This protein shares similarity with 2NXF across 227 of 271 residues and shows a strong Z score of 24.1. The next closest matches were predominantly purple acid phosphatases from various mammalian species including humans, pigs and rats.
| Top 20 Structural Homologs Returned from the Dali Server |
|---|
Protein Architecture
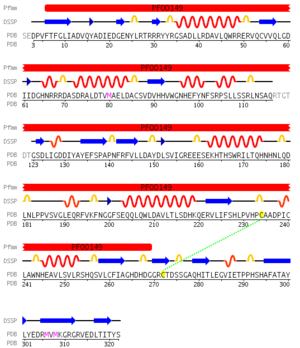
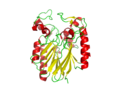
pfam analysis shows a common domain to all these proteins as being a calcineurin-like phosphoesterase (PF00149). SCOP classification of the protein shows it to be an Alpha and Beta (a + b) protein with a 4-layer sandwich fold of alpha/beta/beta/alpha of the Metallo-dependent phosphatases. it belongs to the serine/threonine phosphatase family of proteins. its CATH classification is 3.60.21.10.2.1.1.1.1.
Structure Analysis
The crystal structure of 2NXF shows that it binds 4Zn, PO4 and an ethanol molecule. The PO4 binds in the largest cleft in the protein at an active site that contains two Zn atoms. This site shows three identical residues across the top five protein matches by Dali. This includes the human homolog as well as the Enterbacter aerogenes protein. The sequence of residues is GNH, with a highly conserved acidic amino acid adjacent and down stream of this pattern. This same sequences were placed into the scorecons server to analyse how informative the alignment was; it returned a result of 99.2%, which rates this alignment as very diverse and informative.
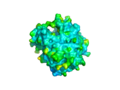
CASTp predicts that the protein contains a number of cavities the largest of which has a volume of 2043.1 covering an area of 950.8. This cavity is very large compared to the others predicted by CASTp. PyMOL analysis of this pocket shows that it is the binding site for the PO4 ligand and contains two Zn atoms buried in the pocket. There are four other cavities of note in this protein, they each have a volume of greater than 200, much higher than the remaining predicted cavities which are less that 100.
The surface view of the 2NXF protein
View of the Binding Pocket with Zn and PO4 ligands